Bridge (chemistry)
In chemical structural formulas, the term bridge describes the linkages of partial structures in molecules through atoms, atomic chains and bonds. However, it has different meanings in the sub-areas of chemistry.
In the IUPAC Compendium of Chemical Terminology ( " Gold Book ") "bridge" is (engl. Bridge defined as follows):
- “ A valence bond or an atom or an unbranched chain of atoms connecting two different parts of a molecule. ”
As linked ( "bridged", engl. Bridged ) are thus atoms in the same molecule, i. H. intramolecular , considered. However, the definition has also been extended to include links between different molecules. H. intermolecular bridges.
According to the IUPAC definition, a bridge can not only consist of atoms or (unbranched) chains of atoms, but can also be a bond, usually a covalent bond. The latter has historical reasons.
Atom bridges - element bridges
Bridges can be clearly classified according to the type of atoms. For example, one speaks of hydrogen bridges, nitrogen bridges, oxygen bridges, sulfur bridges and halogen bridges.

|
|
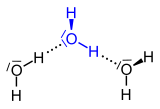
| Three-center bond in diborane | Hydrogen bonds (shown in dotted lines) in water |
Hydrogen as a bridge atom can be found in diborane , aluminum hydride (Alan, (AlH 3 ) x ) and some of their alkyl derivatives. These molecules are also considered to be metal complexes , with the H atoms being referred to as bridging ligands .
Various models have been developed for the non-covalent bonds in these molecules, e.g. B. that of the three-center bond . Another type of bridge that is much more important in nature is the hydrogen bond . It links - in the simplest case - H 2 O molecules or molecules of hydrogen fluoride (HF).

|
|
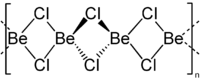
| Aluminum chloride, dimer in the gas phase | Beryllium chloride, chain polymer |
Halogen atoms bridge Al (Hal) 3 structures in aluminum chloride and aluminum bromide , which means that these molecules can exist as dimers. The association comes about through coordinative ties . With beryllium chloride , the chlorine bridges create a chain structure.
Bridges with carbon atoms


Diiron nonacarbonyl , tri- μ -carbonyl-bis (tricarbonyl iron) Dimeric triethylaluminum with three-center bonds
Metal complexes and organometallic compounds
Many metal complexes in inorganic chemistry contain carbon atoms, which can be regarded as parts of bridging ligands. Since C atoms are tetravalent, the structures are more complicated. An alkyl group can bridge metal atoms in complex compounds. Carbonyl bridges can be identified in nonacarbonyl diiron ( diiron nonacarbonyl ) and other “carbonyl complexes”.
Bridging ligands are denoted in the name and in the empirical formula of the substance with the prefix μ n -, where n stands for the degree of bridging (the number of metal centers to which the ligand coordinates). Usually μ 1 is left out and μ 2 is abbreviated to μ . The systematic name of nonacarbonyldiiron is tri- μ -carbonyl-bis (tricarbonyliron), the sum formula [{Fe (CO) 3 } 2 ( μ -CO) 3 ]. The dimeric triethylaluminum is systematically di- μ -ethyl-bis (diethylaluminum) or [(AlEt 2 ) 2 ( μ -Et) 2 ] (Et = C 2 H 5 ).
Hydrocarbons
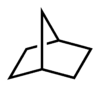
Historically, the term "bridge" was first used to describe the structure of some cyclic hydrocarbons by Adolf von Baeyer . Baeyer viewed hydrocarbons such as norbornane as bridged and developed a nomenclature for bicyclic compounds ( Von Baeyer nomenclature ) from this consideration . While an atomic bridge with the element carbon can be identified in norbornane, there is a covalent bridge in norcaran, which links two carbon atoms. More details in the article Bridged Hydrocarbons . The points of connection of a bridge (the tertiary carbon atoms) are called “ bridgeheads ”. Norbornane is systematically called bicyclo [2.2.1] heptane.
In bi- and polycyclic ring systems, Baeyer also defined bonds as bridges. He called them direct bridges . Since these bridges do not contain an atom, he characterized them by the number zero. Norcaran is therefore bicyclo [4.1.0] heptane. For more examples, see the Von Baeyer nomenclature article .
Other bridged hydrocarbons are the cyclophanes , compounds with one or more benzene rings that are bridged by aliphatic chains and thus form a kind of handle ( ansa compounds ).
Heterocycles
Heterocycles can be formally derived from cyclic hydrocarbons by replacing one carbon atom (or several) with atoms of other elements (“heteroatoms”). Heterocycles can therefore contain atom bridges and also be bridged by covalent bonds. In the case of heterocycles, there are definition problems to differentiate between the terms bridge and functional group or substance class ( substance group ). It is recommended to classify heteroatoms in "simple", i.e. H. monocyclic heterocycles are not to be called bridges, but as functional groups.
The term "bridge" is mainly used to describe the structure of complex molecules from nature ( natural substances and their derivatives) and biologically active substances in pharmaceutical chemistry.
Examples:
The bicyclic tertiary amine tropane (N-methyl-8-aza-bicyclo [3.2.1] octane) and the derivatives derived therefrom, e.g. B. Cocaine , contain a nitrogen bridge through which two carbon atoms of the cycloheptane skeleton are linked.
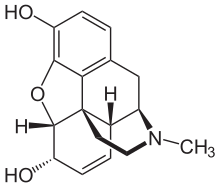
In morphine both a nitrogen bridge and an oxygen bridge exists. Considered as functional groups, these bridges are tertiary amine or ether groups.
The glycosidic bond links carbohydrate molecules with one another or with other structural elements ( aglycon ). The link can be viewed as an oxygen bridge. If this is replaced by a methylene bridge , pharmaceutically interesting “C-glycosides” are obtained.
Bridges in macromolecules
Macromolecules can also be bridged by atoms, mostly chains of atoms. The oldest example is probably the vulcanization of rubber; During this process, sulfur bridges form between the polyisoprene chains.
In biochemistry , bridges in proteins and peptides are important, especially disulfide bridges ( cystine bridges), i. H. Sulfur bridges.
Finally, the term “bridge” was also transferred to ion bonds . Chains and rings of macromolecules can thereby be fixed in certain conformations. In biochemistry, the term “salt bridge” is used for this type of interaction. Salt bridges arise when positively charged functional groups in a protein chain are negatively charged, i.e. H. Electrostatically attract anionic groups (Coulomb interaction). Typical “salt pairs” are guanidinium cations ( arginine peptides) or ammonium groups ( lysine peptides) combined with carboxylate anions (from glutamate building blocks).
See also
literature
- Otto-Albrecht Neumüller (Ed.): Duden - The dictionary of chemical technical expressions. Dudenverlag, Mannheim 2003, ISBN 3-411-04171-4 .
- Entry to bridges. In: Römpp Online . Georg Thieme Verlag, accessed on June 22, 2014.
Individual evidence
- ↑ Entry on bridge . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.B00736 .
- ↑ Nomenclature of Inorganic Chemistry / International Union of Pure and Applied Chemistry (IUPAC). - German edition of the recommendations 1990, edited by W. Liebscher, with the collaboration of J. Neels. VCH, Weinheim u. a. O., 1995, ISBN 3-527-29340-X .
- ↑ Entry on bridging ligand . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.B00741 Version: 2.3.2.
- ↑ Erwin Riedel (Ed.): Modern Inorganic Chemistry . Walter de Gruyter, Berlin / New York, 3rd edition 2007, page 385. ISBN 978-3-11-019060-1 .
- ^ A b Adolf Baeyer: Systematics and nomenclature of bicyclic hydrocarbons. In: Reports of the German Chemical Society 1900, 33, pp. 3771-3775 ( doi: 10.1002 / cber.190003303187 ).

![[7] metacyclophane](https://upload.wikimedia.org/wikipedia/commons/thumb/4/45/7Metacyclophan.svg/130px-7Metacyclophan.svg.png)
![[2.2] paracyclophane](https://upload.wikimedia.org/wikipedia/commons/thumb/4/43/Paracyclophan.svg/150px-Paracyclophan.svg.png)