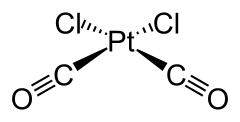
Dicarbonyl dichloroplatinum
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Dicarbonyl dichloroplatinum | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | [PtCl 2 (CO) 2 ] | ||||||||||||
| Brief description |
white solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 326.04 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
103 ° C |
||||||||||||
| solubility |
Decomposes in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Dicarbonyldichloroplatinum is a chemical compound from the group of metal carbonyls .
Extraction and presentation
Dicarbonyldichloroplatinum can be obtained by reacting platinum (II) chloride with carbon monoxide in the absence of moisture, with higher carbonyl halides always being formed.
In analytically pure form it can be obtained by normal pressure carbonylation of hexachloridoplatinic acid in thionyl chloride .
Dicarbonyl dichloroplatinum was the first true heteroleptic metal carbonyl complex to be synthesized. Paul Schützenberger succeeded in presenting it in 1868 by introducing chlorine and carbon monoxide into platinum black .
properties
Dicarbonyldichloroplatinum is a white crystalline solid that is air-sensitive and extremely sensitive to hydrolysis. When exposed to moisture, it quickly turns brown to black with decomposition.
Individual evidence
- ↑ a b c d e f g Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1965.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Schützenberger, P .: Mémoires sur quelques réactions domnant lieu à la production de l'oxychlorure de carbone, et sur nouveau composé volatil de platine . In: Bulletin de la Société Chimique de Paris . 10, 1868, pp. 188-192.

![{\ displaystyle \ mathrm {H_ {2} [PtCl_ {6}] + 3 \ CO \ longrightarrow PtCl_ {2} (CO) _ {2} + COCl_ {2} +2 \ HCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/28ede6489be09fdaf198e91e4bdfdc194d871bcd)