Gas chromatography
The gas-liquid chromatography (GLC) or simply gas chromatography (GC) is both an adsorption as well as a partition , is the wide as an analytical method for separating mixtures into individual chemical compounds used. The GC can only be used for components that can be vaporized in gaseous form or without decomposition (boiling range up to 400 ° C). In this type of chromatography , an inert gas such as nitrogen or helium is used as the mobile phase , and in special cases hydrogen . The carrier gas is pressed through a coiled, curved, capillary-like tube , the so-called column , which is usually 10-200 meters long.
This separation column is either made of metal (in older models), but today it is mainly made of quartz glass , which is coated to increase resistance to breakage. It is lined on the inside with a defined stationary phase (often with viscous polyorganosiloxanes ) and is located in a temperature-controlled oven.
After entering a sample substance, which is now transported by the carrier gas, the components remain on the stationary phase of the column for different lengths of time, depending on the polarity and vapor pressure of the individual gas molecules. A detector measures the time of exit at the end of the column; With a recorder attached to the detector, this point in time and the amount of the substance can be displayed graphically and compared with standard substances. This enables a very quick and easy qualitative and quantitative determination of even very complex substance mixtures. In contrast to high-performance liquid chromatography (HPLC), only sufficiently volatile substances can be detected.
Technology history

The development of gas chromatography was shaped by fundamental work by Archer JP Martin , Erika Cremer and Fritz Prior . The first gas chromatogram in history was created shortly after the Second World War and comes from Erika Cremer's laboratory. It shows the separation of air and carbon dioxide on activated carbon. In 1951, a first gas chromatograph in the current sense was developed by Anthony Trafford James and AJP Martin. Your first published work in 1952 showed the GC separation of carboxylic acids. The development of the electron capture detector (ECD) in 1957 by James E. Lovelock enabled the sensitive detection of chlorinated environmental toxins such as PCB and chlorinated pesticides such as DDT by GC. In the years that followed, a number of companies that offered commercial gas chromatographs emerged, such as the F&M Scientific Corporation of Avondale , Pennsylvania, which was taken over by Hewlett-Packard in 1965 . In the 1970s, the development of capillary gas chromatography took place, a milestone was the invention of the "Fused Silica Column" by Raymond D. Dandenau and Ernest Zerrender. Soon afterwards, the first practical couplings between capillary gas chromatography and mass spectrometry (GC-MS) followed . Today gas chromatography is one of the most important analytical techniques in chemical laboratories.
Measuring principle
The chromatographic separation of a substance mixture in a gas chromatograph takes place in the simplest case with a non-polar carrier column exclusively on the basis of the different boiling points of the individual substances in the mixture, whereby there is no special interaction with the stationary phase, but "only" ten thousand times repeated adsorption and desorption . With polar separation columns, however, alcohols, esters, ketones with the same boiling points as similar paraffins are retained more strongly. The special interaction - more precisely the equilibrium between the gas phase and the stationary phase - is known as Raoult's law . The higher the partial vapor pressure of a substance according to Raoult's law, i. That is, the longer the substance is in the gas phase, the shorter the retention time .
The strength of the interactions between the sample components and the stationary phase is determined both by their structure and by their functional groups. In the case of non-polar substances, only dispersion interactions ( van der Waals bonds ) occur, while polar separation phases can also enter into polar interactions, e.g. B. hydrogen bonds or donor-acceptor bonds. The latter separate according to the principle: opposites attract. This means that separation phases, which z. B. are able to take up hydrogen for hydrogen bonding are able to separate substances that can provide hydrogen for bonding (e.g. alcohols). Enantiomers , for example , which do not differ in their boiling points and would therefore have the same retention times, can also be separated due to their interactions of different strengths with special derivatives of cyclodextrins .
A basic requirement for gas chromatography is that the substance that is to be examined can be vaporized without being decomposed - provided it is not already in gaseous form. By means of derivatisation , analytes that are otherwise difficult to access, such as alcohols, amines, fatty acids or sugars, can be thermally stabilized to such an extent that they can be separated into commercially available phases without difficulty. Possible derivatives for carboxylic acids are the methyl esters (conversion with BF 3 and MeOH), for alcohols the silyl ethers .
Important parts of the device
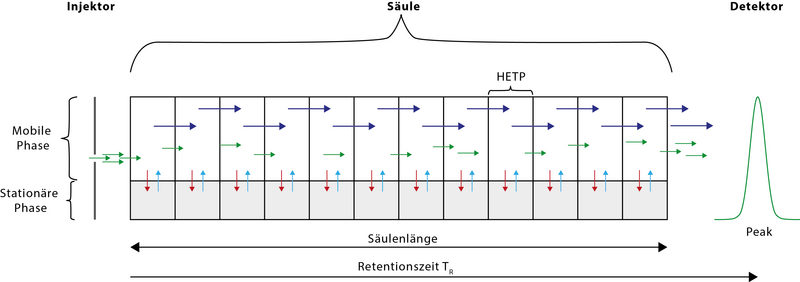
A gas chromatograph consists of three main components: injector, separation column in the GC oven and detector. In the injector , the sample, dissolved in a low-boiling solvent, is injected through a puncture membrane (septum). This injector is usually heated (up to 450 ° C) in order to achieve rapid and complete evaporation of the sample. The septum-free feed and slow evaporation using a cold feed system (KAS / PTV) are also possible. The substances are transported by the carrier gas (column pressure normally up to 6 bar) into the separation column (capillary), which is built into the so-called GC oven. This is used to precisely control the temperature of the separation column in order to achieve a rapid and extensive separation of the mixture of substances through constant temperature (isothermal) or through a controlled increase in temperature.
At the end of the column there is the detector , which generates an electronic signal when a substance leaves the separation system. The electronic signal can be registered as a peak on the recorder. The signals are then processed on an integrator or today mostly on a computer system with appropriate evaluation software. The time it takes to separate a mixture of substances and display the various peaks in a chromatogram is often between 30 and 60 minutes.
Injectors
The injector is used to apply the substance mixture to be examined to the separation column. Common injectors / methods are:
- Split / Splitless Injectors ( SSL )
- On-column injections ( OCI ) with direct injection onto the column
- Cold infeed systems (depending on the manufacturer, KAS or PTV )
- Direct feed systems by means of valve circuits
For packed columns the optimal amount of sample per component is between 0.1 and 1 μl, for capillary columns the optimal amount of sample should be 100 to 1000 times smaller. There are special 1–10 μl syringes for injecting a sample that can also be diluted with a solvent. It is important for the injection that there are no air (bubbles) in the syringe, because this would contribute to the oxidation of the substances in the furnace chamber.
So-called autosamplers , which allow the sequential processing of a large number of samples, are often used with split / splitless injectors and cold injection systems. In addition, u. a. Headspace samplers, purge & trap systems and pyrolysers are also used for sample introduction. A fairly new development is the use of solid phase micro-extraction (SPME) or stir bar sorptive extraction (SBSE).
Used separation columns
Important parameters of the separation columns are:
- the column diameter
- the column length
- and the type of stationary phase (occupancy).
In the past, mostly packed columns were used. Inside a thin (<1 cm) metal or glass tube a few meters long, the so-called column, is a solid, inert carrier material. If the gas with the substance to be analyzed is passed directly over the carrier material, one speaks of GSC ("Gas-Solid-Chromatography"). If the carrier substance is also coated with a thin layer of a high molecular weight, viscous and not very volatile liquid, it is a GLC ("Gas-Liquid-Chromatography"). This liquid takes on the function of the actual stationary phase here. The inert gas preferably used here is nitrogen .
In the last few decades, the capillary gas chromatography developed by Marcel JE Golay in 1958 has been used predominantly . The advantage is the approx. 100-1000 times better separation (a number of separation stages of approx. 300,000) of substances compared to packed columns, so that the analysis time can also be shortened. The quartz glass separation columns coated with polyimide on the outside for stabilization normally have an inside diameter of 0.1 to 0.5 mm and a length of 10 to 60 m. Combined columns up to 100 m are even used to separate fatty acid esters. The stationary phase only lines the capillary as a thin film, usually 0.1 to 5 µm. The advantage is the drastically better separation of similar substances compared to packed columns. The trend in GC is currently towards ever thinner and shorter columns, because this can significantly reduce the time required for analyzes. The inert gas used with preference is helium , but hydrogen or, more rarely, nitrogen are also used.
An important criterion for classifying the stationary phase is the polarity of its occupancy. Analytes on non-polar columns (type DB-1 or similar) usually elute according to their boiling points. Polar compounds, on the other hand, interact selectively with the polar stationary phases in polar columns and are retained longer according to their polarity and elute later than comparable non-polar compounds such as e.g. B. alkanes . When using columns from different manufacturers, it should be noted that identical stationary separation phases are given a wide variety of names. The following list of common stationary separation phases shows which separation columns from different manufacturers are comparable with regard to the composition of the occupancy of the separation columns.
| Composition of the occupancy of the separation column |
Manufacturer designation | Temperature range | polarity |
|---|---|---|---|
|
Polydimethylsiloxane 100% methyl |
DB-1, SB-1, BP-1, CP-Sil 5 CB, OV-1, OV-101, PB-1, SPB-1, RTX-1, PE-1, Ultra 1, ZB-1, AT -1, SE-30 | −50 ° C to +350 ° C | non-polar |
|
Polyphenylmethylsiloxane 5% phenyl, 95% dimethyl |
DB-5, SB-5, BP-5, CP-Sil 8 CB, PVMS-5, PB-2, SPB-5, Rtx-5, PE-2, Ultra 2, ZB-5, AT-5, SE -54, Optima-5, RSL-200 | −20 ° C to +350 ° C | slightly polar |
|
Polyphenylmethylsiloxane 14% phenyl, 86% dimethyl |
DB-624, SB-624, CP-Sil 13 CB, VOCOL, Rtx-Volatiles, PE-502, AT-62 | ± 0 ° C to +250 ° C | medium polar |
|
Polycyanopropylphenylmethylsiloxane 6% cyanopropylphenyl, 94% dimethyl |
DB-1301, SB-1301, Rtx-1301, AT-1301 | ± 0 ° C to +230 ° C | polar |
|
Polyphenylmethylcyanosiloxane 6% phenyl, 6% cyano, 88% methyl |
DB-1701, SB-1701, BP-10, CP-Sil 19 CB, PB-1701, SPB-7, Rtx-1701, PE-1701, PAS-1701, AT-1701, RSL-300 | −50 ° C to +225 ° C | polar |
| Polyethylene glycol | DB-WAX, SB-Wax, BP-20, CP-Wax 52 CB, Supelcowax-10, Stabilwax, PE-CW, HP-20M, AT-Wax | ± 0 ° C to +220 ° C | polar |
| Polyethylene glycol-2-nitroterephthalic acid ester | OPTIMA FFAP, DB-FFAP, HP-FFAP, CP-Sil 58 CB, 007-FFAP, CP-FFAP, Nukol | ± 0 ° C to +250/260 ° C | polar |
Since the beginning of the 1990s, column manufacturers have made great efforts to develop low-bleeding columns that are primarily used in GC-MS . These can often be recognized by the additional designation "-ms".
Separating columns which are covered with a chiral stationary phase can be used for enantiomer analysis. This allows the enantiomeric ratio to be determined in enantiomeric mixtures and the analysis of the enantiomeric purity of supposedly pure enantiomers.
Detectors
The following detectors are used:
- Flame ionization detector (FID), generally for the quantification of organic compounds, is the most widely used detector in gas chromatography
- Thermal conductivity detector (TCD or TCD), for permanent gases
- Photoionization Detector (PID)
- Flame photometric detector (FPD), element-specific
- Nitrogen-phosphorus detector (English NPD, also thermionic detector , TID), nitrogen-phosphorus-specific
- Electron capture detector (ECD), for halogenated organic compounds
- Pulsed charge detector (English PD), also for halogenated organic compounds
- Atomic Emission Detector (AED), element specific
- Echelle plasma emission detector (EPED), element-specific
- Mass spectrometer , mass selective detector (MS or MSD)
- Ion mobility spectrometer (IMS) for volatile organic compounds
- Odor detector (human nose)
In some cases, two (or more) detectors are connected in series for special issues (tandem principle). The basic requirement for this, however, is that the front detectors do not carry out the measurement in a destructive manner (e.g. an ECD / TLD, but not an FID / NPD).
The electronic signals of the detector are displayed as a 2D graph, the so-called chromatogram , as a function of the time since the injection (retention time) . This is usually done with the help of electronic evaluation units (computers with chromatography data system), rarely only with plotters.
Application in analytics
Gas chromatography is a very sensitive method for analyzing mixtures of substances. Even minimal amounts of substance (10 −9 grams) can be detected. It can be used to separate complex mixtures of substances into individual components. In many cases, the retention time alone is sufficient for a substance to be identified by the time it takes for a substance from the time of injection to passing through the detector . By combining it with a mass spectrometer , the so-called GC / MS coupling, very small amounts of substances can be detected and structure elucidation can be carried out at the same time .
Gas chromatography is used in the analysis of agricultural products for herbicides , meat products for hormones, the examination of drugs , aromas and essential oils, carbohydrates , petroleum components and in forensic chemistry, in doping tests, in air and seawater tests in environmental analysis . Even high molecular substances such as triglycerides and waxes can be separated, identified and quantified on temperature-stable stationary silicone phases. Hardly volatile analytes may have to be derivatized before analysis, i.e. converted into more volatile substances. This can e.g. B. done with trimethylsulfonium hydroxide or chlorotrimethylsilane by converting polar groups into non-polar methylated groups.
Use in quantitative analysis
The size of the area units displayed by the detector is rarely directly related to the actual mass fractions in the sample to be analyzed. This makes calibration with reference materials of a defined content necessary. In order to rule out (accidental) errors in the gas chromatograph (especially in the injection system), internal standards are often used , especially in gas chromatography . An additional substance serves as an internal standard here, the retention time of which is close to the analyte to be determined, but does not superimpose it. It is added to the analyte and the reference material or the solutions made from it. After the measurement, the respective peak areas of analyte and reference material are set in relation to the peak area of the internal standard and the errors of the injection system are calculated out as far as possible. After calibration, the concentration c P of a sample P can be determined using the following equation.
c S is the weighed-in concentration of standard, A denotes the area of the peak in the chromatogram and r denotes the sensitivity of a substance (how much signal the detector shows per M). r can be measured once for a specific detector and then tabulated.
literature
- Ernst Bartholomé et al. (Ed.): Ullmanns Encyklopadie der technischen Chemie . Volume 5: Hans Kelker (Ed.): Analysis and measurement methods. 4th, revised and expanded edition. Verlag Chemie, Weinheim et al. 1980, ISBN 3-527-20005-3 , pp. 118 ff.
- Eberhardt Leibnitz, Hans Georg Struppe (Hrsg.): Handbook of gas chromatography. 3rd, revised and greatly expanded edition. Academic publishing company Geest & Portig KG, Leipzig 1984, ISBN 3-89141-001-8 .
- Gerhard Schomburg : gas chromatography. Basics, practice, capillary technology. 2nd, revised and expanded edition. VCH, Weinheim et al. 1987, ISBN 3-527-26461-2 .
- Reiner Wittkowski , Reinhard Matissek (Eds.): Capillary Gas Chromatography in Food Control and Research , B. Behr's Verlag GmbH & Co., Hamburg 1992, ISBN 3-86022-037-3 .
- Peter J. Baugh (Ed.): Gas Chromatography. A user-oriented presentation. Vieweg, Braunschweig et al. 1997, ISBN 3-540-67009-2 .
- Walter David, Burkhard Kusserow: GC tips. Problem solutions for everything to do with the gas chromatograph. Hoppenstedt, Darmstadt 1999, ISBN 3-8203-0469-X .
- Bruno Kolb: Gas Chromatography in Pictures. Wiley-VCH, Weinheim et al. 1999, ISBN 3-527-29880-0 .
- Keith D. Bartle, Peter Myers: History of gas chromatography. In: Trends in Analytical Chemistry . Vol. 21, No. 9/10, September 10, 2002, pp. 547-557, doi : 10.1016 / S0165-9936 (02) 00806-3 .
Web links
- ETH Zurich, Analytical Chemistry: Gas Chromatography (GC) (PDF; 991 kB)
- A simulation is implemented in NetLogo .
Individual evidence
- ↑ cf. LC GC International , Vol. 3 No. 11.
- ↑ AT James, AJP Martin: Gas-liquid partition chromatography: the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid . In: Biochemical Journal . 50, 1952, pp. 679-690.
- ^ JE Lovelock: A sensitive detector for gas chromatography . In: Journal of Chromatography A . 1, No. 1, 1958, pp. 35-46. doi : 10.1016 / S0021-9673 (00) 93398-3 .
- ^ JE Lovelock: The electron capture detector . In: Journal of Chromatography A . 99, No. 1, 1974, pp. 3-12. doi : 10.1016 / S0021-9673 (00) 90840-9 .
- ↑ Dandenau, Raymond D. and EH Zerenner: An investigation of glasses for capillary chromatography . In: Journal of High Resolution Chromatography . 2, No. 6, 1979, pp. 351-356. doi : 10.1002 / jhrc.1240020617 .
- ↑ Infographic - The Incredible Progress in Analytical Chemistry. In: German paint institute. Retrieved August 10, 2019 .
- ↑ Dioxin Analysis. In: Federal Institute for Risk Assessment. Retrieved August 10, 2019 .
- ↑ Wolfgang Brodacz, Marc Platthaus: Comparison and combination of GC phases. LaborPraxis, 4/2004.
- ↑ N. Limsathayourat, H.-U. Melchert: High-temperature capillary GLC of hydrocarbons, fatty-acid derivatives, cholesterol esters, wax esters and triglycerides in beeswax analysis. In: Fresenius' Journal of Analytical Chemistry. 318, No. 6, 1984, pp 410-413, doi : 10.1007 / BF00533223 .
- ↑ Uri Wilensky: NetLogo Models Library: Gas Chromatography. Retrieved November 27, 2018 .