Solid phase micro-extraction
Solid phase microextraction (English solid phase microextraction , SPME ) is a method of sampling and analyte enrichment, which in some areas of chemical analysis is advantageous compared to classical methods such. B. "purge and trap" or SPE ( S olid P hase E Xtraction, solid phase extraction) has been established. It has been developed by Janusz Pawliszyn since 1990.
Worldwide the sole licensee for solid phase microextraction technology is Supelco (US patent 5,691,206; European patent EP523092).
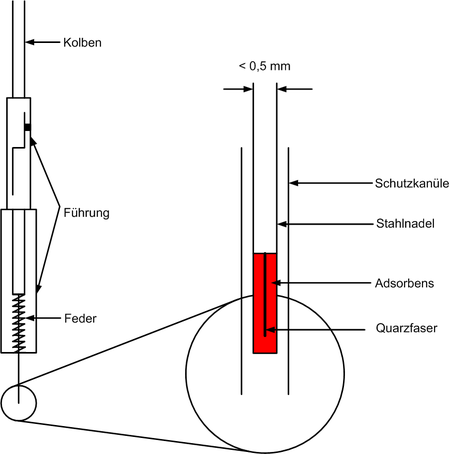
Structure of a sampler
A schematic illustration (below, not to scale) shows the structure of an SPME sampler.
The sampler essentially consists of four parts:
- A guide for a piston. The guide has a bayonet lock to lock the piston in the depressed state. The two separate parts of the guide can be adjusted relative to one another by means of a thread, in order to enable the protective cannula to penetrate a sample vessel to a greater or lesser extent.
- A piston to extend the sampler. At the end of the piston there is a spring that pushes the piston back when the lock is released.
- The actual sampler screwed to the piston. It consists of a stainless steel needle to which a quartz fiber (“fused silica”) or glass fiber (“StableFlex”) is attached. This quartz fiber is covered with a thin layer of adsorbent .
- A protective cannula through which the fiber is guided due to its poor mechanical stability.
sampling
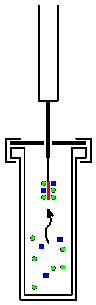
First, the protection is with the needle piercing the membrane (septum) of the sample vessel pierced. The fiber is then extended by pressing down the punch. Once the adsorbent is exposed, molecules of the analyte are adsorbed . This leads to a strong concentration of the analytes relative to the sample. As a first approximation, the amount adsorbed is linearly proportional to the concentration in the sample. The figure below shows adsorption from the gas phase (“headspace sampling”). This can also be done analogously with adsorption from the liquid phase (“immersion sampling”).
After equilibrium has been established, the locking of the piston is released and the fiber is withdrawn into the protective cannula, whereby the adsorption is interrupted. Then the sampler is removed. The amount adsorbed hardly changes in the retracted state, even in the course of hours, which enables samples to be easily transported to a laboratory. The actual analysis then takes place. To do this, the sampler is pushed through the septum into the injector of a gas chromatograph . Then the fiber is extended. Due to the high temperature in the injector is it, then the desorption of the analytes. The fiber is then retracted and the sampler removed. The desorption can alternatively take place by extraction in a modified injector port of an HPLC device.
Analogy to nature
The snakes' sense of smell works in a very similar way in nature: the tongue shoots out of the mouth, odorous substances are adsorbed by the moist surface, the tongue is withdrawn into the mouth, the odorous substances are transferred to Jacobson's organ and only analyzed there.
Reusability
If handled carefully, a fiber can be used for at least 50 analyzes without problems. This assumes that the fiber is not mechanically (overloaded) stressed, it is cleaned regularly by heating it in the injector of a gas chromatograph or a suitable conditioning station and it does not come into contact with aggressive chemical solvents that attack the adsorbent.
Sampling conditions
The optimization of the sampling conditions requires the variation of various parameters. The general principle always applies that good results can only be achieved by carefully following good methods.
Adsorbent
Commercially five adsorbents (highest polarity : polyacrylate , Carbowax , polydivinylbenzene , Carboxen and PDMS , P oly d i m ethyl s iloxan) available in several combinations and layer thicknesses for different applications. The rule of thumb is that polar analytes should be analyzed with polar adsorbents and non-polar analytes with non-polar adsorbents. Thinner layers are recommended for high-molecular analytes.
Adsorption time
The time- adsorbate curve is not linear, but asymptotic. For reproducible measurement results you should work close to the equilibrium setting. If different analytes occur in strongly changing or very high concentrations, displacement phenomena can occur on the fiber. Under these circumstances it is also possible to work in pre-equilibrium, but then it is essential to ensure precise time control. When trace analysis is usually required longer adsorption times.
Adsorption conditions
For headspace analysis of non-volatile compounds from solid or semi-solid samples, the sample vessel is usually heated in a temperature-controlled heating block. Temperatures in the literature vary from 40 to 65 ° C . In the SPME analysis of liquid samples, the extraction can be improved by setting a suitable pH value and by saturating them with common salt . It is absolutely necessary to stir the sample intensively (250 / min ) in order to compensate for the lower diffusion coefficients compared to the gas phase . The stirring speed must also be kept constant. This applies to measurements via headspace as well as via immersion.
Desorption conditions
Desorption usually takes place in the injector of a gas chromatograph at temperatures between 200 and 280 ° C. Five minutes are usually sufficient. A check can be carried out by extending the desorption under otherwise constant adsorption conditions and plotting the integrals of analyte peaks against the desorption time. If a parallel to the X-axis is obtained, the desorption is complete. Proceed in the same way for desorption in the injector port of an HPLC system.
advantages
Although one could think differently after the above, the time required to optimize the sampling conditions is more than outweighed by the drastic reduction in the time required for sampling. Automation is also possible. Overall, this saves a considerable amount of time (> 70% per sample) for routine analyzes.
Compared to liquid / liquid extraction, the complete renunciation of organic solvents means a considerable ecological and economic advantage, since no subsequent disposal of the solvents is necessary. In addition, the risk of cross-contamination by solvents is eliminated.
Dispensing with time-consuming sample preparation means that fewer samples are changed. This is particularly important with sensitive analytes. SPME can be used for both gaseous and liquid samples . In terms of breadth, this does not apply to “purge and trap” or liquid-liquid extraction . A method similar to SPME is the stir bar sorptive extraction .
disadvantage
Because of the small amounts that are adsorbed by the fiber, this method is suitable, unlike e.g. B. SPE, not for the preparative isolation of the analytes. The achievable enrichment factors are also lower compared to the SPE or SBSE.
Areas of application
- Environmental analysis (e.g. determination of herbicides in drinking water and pesticides in blood plasma and urine )
- Food analysis (e.g. determination of light-generated breakdown products in milk)
- Aroma analysis (e.g. from geosmin , determination of the odorous substances in honey and flower scent In a special case literally recording the scent of a single flower on board the space shuttle )
- Forensics (e.g. determination of amphetamines in urine)
- Sample application in gas chromatography (special headspace technique)
literature
- C. Arthur, J. Pawliszyn: Solid phase microextraction with thermal desorption using fused silica optical fibers. In: Anal. Chem. 62, 1990, pp. 2145-2148, doi: 10.1021 / ac00218a019 .
- J. Pawliszyn: Solid Phase Microextraction - Theory and Practice. Wiley-VCH, New York / Weinheim 1997, ISBN 0-471-19034-9 .
Web links
- additional application examples (PDF; 2.53 MB)
Individual evidence
- ↑ Q. Wu, C. Feng, G. Zhao, C. Wang, Z. Wang: Graphene-coated fiber for solid-phase microextraction of triazine herbicides in water samples. In: J Sep Sci. Dec 8, 2011. PMID 22162195
- ↑ L. Gao, J. Liu, C. Wang, G. Liu, X. Niu, C. Shu, J. Zhu: Fast determination of paraquat in plasma and urine samples by solid-phase microextraction and gas chromatography-mass spectrometry. In: J Chromatogr B Analyt Technol Biomed Life Sci. 944, Jan 1, 2014, pp. 136-140. PMID 24316524
- ↑ Z. Ding, S. Peng, Y. Jin, Z. Xuan, X. Chen, L. Yin: Geographical and seasonal patterns of geosmin and 2-methylisoborneol in environmental water in jiangsu province of china. In: Methods Chem. 2014, p. 743924. PMID 25400979
- ↑ S. Seisonen, E. Kivima, K. Vene: Characterization of the aroma profiles of different honeys and corresponding flowers using solid-phase microextraction and gas chromatography-mass spectrometry / olfactometry. In: Food Chem. 169, Feb. 15, 2015, pp. 34-40. PMID 25236195
- ↑ I. Racamonde, R. Rodil, JB Quintana, R. Cela: In-sample derivatization-solid phase microextraction of amphetamines and ecstasy related stimulants from water and urine. In: Anal Chim Acta. 770, Apr 3, 2013, pp. 75-84. PMID 23498689