Minisci reaction
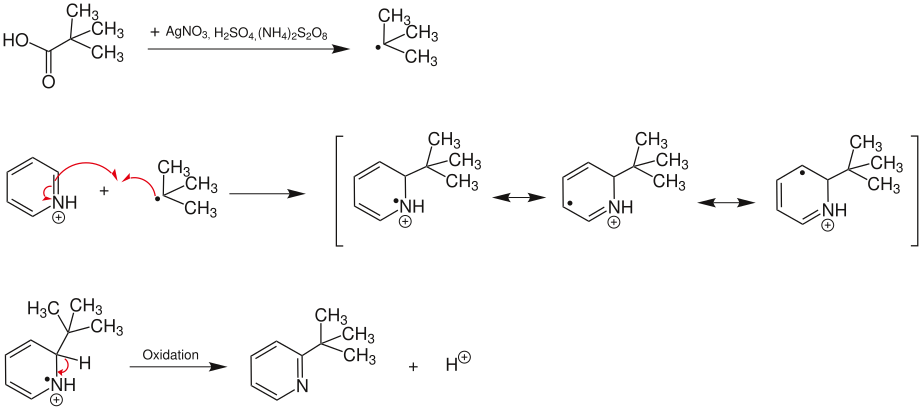
The Minisci reaction is a name reaction in organic chemistry . It is a radical substitution on aromatics , preferably on heteroaromatics , which serves to introduce alkyl radicals . It was first published in 1971 by Francesco Minisci . The Minisci reaction is regioselective and enables the introduction of a wide range of alkyl groups. As essential side reaction a running acylation from. The ratio between alkylation and acylation depends on the substrates and the reaction conditions. Due to the simple starting materials and the simple way of carrying out the reaction, the Minisci reaction has many areas of application in heterocycle chemistry .
mechanism
First, an alkyl radical is formed from the carboxylic acid used . This is done by oxidative decarboxylation with silver salts and an oxidizing agent . The oxidizing agent is used to reoxidize the silver salt . The resulting radical then adds to the aromatics. The desired product is released through rearomatization . The acyl radicals that form as a side reaction lead to the formation of acylated products.
Individual evidence
- ^ F. Minisci, R. Bernardi, F. Bertini, R. Galli, M. Perchinummo: Nucleophilic character of alkyl radicals — VI: A new convenient selective alkylation of heteroaromatic bases , in: Tetrahedron 1971 , 27 , 3575-3579. doi: 10.1016 / S0040-4020 (01) 97768-3
- ^ A b F. Fontana, F. Minisci, MCN Barbosa, E. Vismara: Homolytic acylation of protonated pyridines and pyrazines with α-keto acids: the problem of monoacylation , in: J. Org. Chem. 1991 , 56 , 2866– 2869; doi : 10.1021 / jo00008a050 .
- ↑ a b M.-L. Bennasar, T. Roca, R. Griera, J. Bosch: Generation and Intermolecular Reactions of 2-Indolylacyl Radicals , in: Org. Lett. 2001 , 3 , 1697-1700; doi : 10.1021 / ol0100576 .
- ↑ PB Palde, BR McNaughton, NT Ross, PC Gareiss, CR Mace, RC Spitale, BL Miller: Single-Step Synthesis of Functional Organic Receptors via a Tridirectional minisci reaction , in: Synthesis 2007 , 15 , 2287-2290; doi : 10.1055 / s-2007-983792 .
- ↑ JA Joules, K. Mills: Heterocyclic Chemistry , 5th Edition, pp. 125-141, Blackwell Publishing, Chichester, 2010, ISBN 978-1-405-19365-8 .
- ↑ László Kürti and Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 290-291, ISBN 978-0-12-429785-2 .