Zincke reaction
The Zincke reaction is a name reaction from the field of organic chemistry . It is used for the synthesis of pyridinium compounds from pyridines and is named after its discoverer, the German chemist Theodor Zincke (1843–1928). It is not to be confused with the Zincke-Suhl reaction or the Zincke nitration .
mechanism
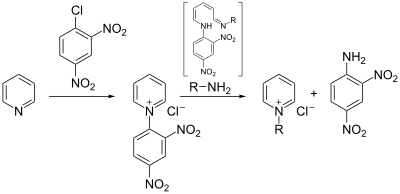
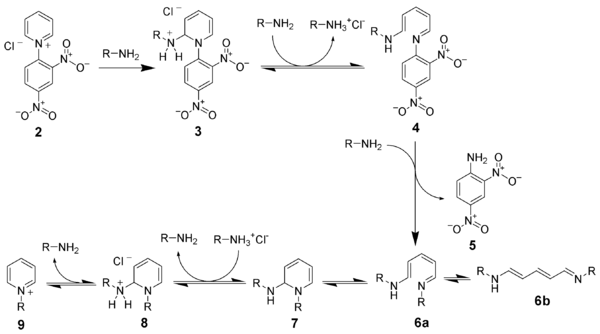
In a first step, a Zincke salt ( 2 ) is produced from the pyridine derivative used. This is done by reacting the pyridine with an electron-poor aromatic , often 1-chloro-2,4-dinitrobenzene , with a pyridinium salt being formed. The salt is then reacted with two equivalents of the primary amine , which carries the residue that should remain on the pyridine after the end of the reaction. The amine initially adds to the pyridinium salt ( 3 ) in the 2-position , which leads to the opening of the ring after deprotonation ( 4 ). By substituting the amine from the previous pyridine with a third equivalent of the desired amine, the desired residue is brought into the correct position ( 6a ). The reaction proceeds as a reversal of the previous course of the reaction. The ring is closed again ( 7 ), the amine in the 2-position is protonated ( 8 ) and the desired pyridinium compound ( 9 ) is released with elimination of the amine .
If secondary amines are used instead of primary amines, open-chain aldehydes , so-called Zincke aldehydes , are obtained from (6) in the presence of water .
Errata
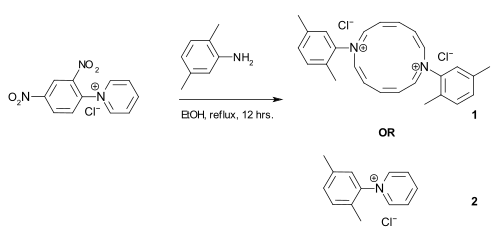
The publication of an alleged one-pot synthesis of diazaannulene ( 1 ), which was published independently by two working groups, has attracted attention in the more recent specialist literature . Thereupon the German university professor Manfred Christl , a former student of R. Huisgen and S. Hünig , wrote a letter to the editor in which he validly questioned the structure and synthesis of the postulated diazaannulene. He also pointed out that the actual reaction that was taking place was the Zincke reaction, which has been known for over a hundred years, and that the corresponding product of the Zincke reaction ( 2 ) would be formed. Both groups then withdrew their publications. The case of recent sloppy scientific research was also reported in Spiegel and Nature . It turned out that both working groups lacked the necessary experience in the evaluation of mass and NMR spectra, techniques that did not yet exist at the time of Zincke. In Zincke's time it was common practice to check molar masses by cryoscopy .
Application in synthesis planning
In addition to the variable synthesis possibility alkyl - or aryl -substituted pyridinium salts is the Zincke reaction also used in recent publications on current problems. For example, a new indole synthesis based on the Zincke reaction was developed.
Individual evidence
- ↑ T. Zincke , G. Heuser, W. Moller: Ueber Dinitrophenylpyridiniumchlorid and its conversion products. , in: Liebigs Ann. 1904 , 330 , 361-374; doi: 10.1002 / jlac.19043300217 and 1904 , 333 , 296-345; doi: 10.1002 / jlac.19043330212 . - T. Zincke , G. Weisspfenning: About Dinitrophenylisoquinolinium chloride and its conversion products. , in: Liebigs Ann. 1913 , 396 , 103-131; doi: 10.1002 / jlac.19133960107 .
- ^ JJ Lie: Name Reactions: A Collection of Detailed Mechanisms , 4th edition, p. 596, Springer Verlag, Berlin, 2009, ISBN 3-642-01052-0 .
- ↑ T. Zincke , W. Wurker: About Dinitrophenylpyridiniumchlorid and its conversion products (2nd communication.). About dinitrophenylpyridinium chloride and its conversion products. , in: Liebigs Ann. 1904 , 338 , 107-141; doi: 10.1002 / jlac.19043380107 .
- ↑ I. Yamaguchi, Y. Gobara, M. Sato: One-Pot Synthesis of N-Substituted diaza [12] annulenes. , in: Org. Lett. 2006 , 8 , 4279-4281; doi: 10.1021 / ol061585q . - L. Shi, D. Lundberg, DG Musaev, FM Menger: [12] Annulene Gemini Surfactants: Structure and Self-Assembly , in: Angew. Chem. 2007 , 119 , 5993-5995; doi: 10.1002 / anie.200702140 .
- ↑ Prof. Dr. Christl - Curriculum Vitae. University of Würzburg , accessed on January 3, 2018 .
- ↑ M. Christl: 1,7-Diaza [12] annulene derivatives? 100 year old pyridinium salts! , in: Angew. Chem. 2007 , 119 , 9312-9313; doi: 10.1002 / anie.200704704 cube .
- ^ Friedrich Konrad Beilstein, Bernhard Prager, Paul Jacobsen: Beilstein's manual of organic chemistry . Ed .: German Chemical Society. 4th edition. tape 5 : cyclic hydrocarbons . Springer, Berlin 1922, p. 264 ( Textarchiv - Internet Archive ).
- ^ Friedrich Konrad Beilstein, Bernhard Prager, Paul Jacobsen: Beilstein's manual of organic chemistry . Ed .: German Chemical Society. 4th edition. tape 20 : heterocyclic series . Springer, Berlin 1935, p. 217 ( Textarchiv - Internet Archive ).
- ↑ I. Yamaguchi, Y. Gobara, M. Sato: One-Pot Synthesis of N-Substituted diaza [12] annulenes. , in: Org. Lett. 2007 , 9 , 5139; doi: 10.1021 / ol702583k . - L. Shi, D. Lundberg, DG Musaev, FM Menger: Correction: [12] Annulene Gemini Surfactants: Structure and Self-Assembly , in: Angew. Chem. 2007 , 119 , 9295; doi: 10.1002 / anie.200790248 .
- ^ Jens Lubbadeh: Science farce: Unsuspecting chemists discover connection for the second time. In: Spiegel Online . December 6, 2007, accessed January 3, 2018 .
- ↑ K. Sanderson: Where have I seen that before? 103-year-old chemical reaction pops up again. , in: Nature News December 2007 ; doi: 10.1038 / news.2007.341
- ↑ AM Kearney, CD Vanderwal: Synthesis of Nitrogen Heterocycles by the Ring Opening of Pyridinium Salts. , in: Angew. Chem. 2006 , 118 , 7967-7970; doi: 10.1002 / anie.200602996 .