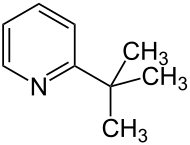
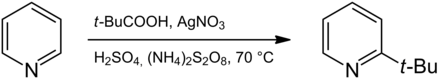2- tert- butylpyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2- tert- butylpyridine | |||||||||||||||
| other names |
2- (1,1-dimethylethyl) pyridine |
|||||||||||||||
| Molecular formula | C 9 H 13 N | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 135.21 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
169 ° C (991 hPa ) |
|||||||||||||||
| solubility |
poor in water (1.8 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.4891 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2- tert -Butylpyridine is an organic compound that belongs to the heterocycles (more precisely: heteroaromatic compounds ). It consists of a pyridine ring with a tert-butyl radical in the 2-position .
presentation
The compound can be prepared from pyridine by radical substitution in a Minisci reaction . For this purpose, pyridine is reacted with pivalic acid , silver nitrate and ammonium peroxodisulfate in a sulfuric acid solution. A tert- butyl radical is generated from pivalic acid , which then reacts with the pyridine ring in the 2-position with high selectivity. The product is obtained with an excellent yield of 97%.
Individual evidence
- ↑ a b c d H. C. Brown, WA Murphey: A Convenient Synthesis of the Monoalkylpyridines; a New Prototropic Reaction of 3-Picoline , in: J. Am. Chem. Soc. , 1951 , 73 , pp. 3308-3312; doi : 10.1021 / ja01151a093 .
- ↑ HP Hopkins Jr., DV Jahagirdar, PS Moulik, DH Aue, HM Webb, WR Davidson, MD Pedley: Basicities of the 2-, 4-, 2,4-Di-, and 2,6-Disubstituted tert-Butyl Pyridines in the Gas Phase and Aqueous Phase: Steric Effects in the Solvation of tert-Butyl-Substituted Pyridines and Pyridinium Cations , in: J. Am. Chem. Soc , 1984 , 106 , pp. 4341-4348; doi : 10.1021 / ja00328a007 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- Jump up ↑ JA Joules, K. Mills: Heterocyclic Chemistry , 5th Edition, Blackwell Publishing, Chichester, 2010, ISBN 978-1-4051-9365-8 , pp. 125-141.