Pyridinium compounds
Pyridinium compounds are a group of chemical compounds in which the nitrogen atom of the pyridine -ring quaternized is. They belong to the quaternary ammonium compounds . Depending on the radical (R) located on the nitrogen atom, the pyridinium compounds can be divided into different categories. Furthermore, the hydrogen atoms of the ring can be substituted . Important representatives are the drug cetylpyridinium chloride , the well-known pesticide paraquat and the coenzyme nicotinamide adenine dinucleotide .
Categories and representatives
Pyridinium compounds can initially be divided into different categories by the remainder that is bonded to the nitrogen atom of the ring. Their representatives then differ in the length of the alkyl radical and the substituents on the ring.
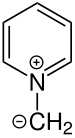
Alkyl pyridinium compounds
| Alkyl pyridinium compound |
|---|
Alkylpyridinium compounds are characterized by the alkyl radical on the ring nitrogen atom. Furthermore, the hydrogen atoms of the ring can be substituted . The simplest representative - methylpyridinium chloride - is given as an example.
N -Ylide
| N -Ylid |
|---|
The N -Ylides are obtained by deprotonating the alkylpyridinium compounds . These are important intermediates in organic synthesis.
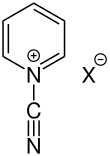
N -cyanopyridinium salts
| N -cyanopyridinium salt |
|---|
N -cyanopyridinium compounds are characterized by the electron-withdrawing radical. This allows nucleophiles to attack easily, causing ring opening to occur. The resulting open-chain aldehydes occur in connection with the Zincke reaction and are referred to as Zincke aldehydes. The carbon atoms of the ring can be substituted and halide ions are present as anions (X - ) .
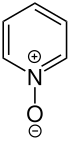
Pyridinium N -oxides
| Pyridinium N oxide |
|---|
Pyridinium – N –oxides are characterized by the fact that they enter into electrophilic as well as nucleophilic substitutions in the ortho and para positions . They are particularly suitable as intermediates for secondary substitutions of pyridine derivatives. The oxygen atom can then be split off with phosphorus trichloride, for example .
synthesis
For example, pyridinium compounds can be synthesized from pyridine by the Zincke reaction :
Applications
Medicinal substances
| Structural formula of the pyridinium compound | Application example |
|---|---|

Treatment of viral pharyngitis
|
Probably the best-known pyridinium compound is cetylpyridinium chloride, which is used as a monopreparation Dobendan or combined preparation Dobendan Strepsils Dolo against infections and inflammation of the throat.
| Structural formula of the pyridinium compound | Application example |
|---|---|

Medication of myasthenia gravis .
|
Pyridostigmine bromide also acts as a drug against the autoimmune disease myasthenia gravis, which manifests itself, for example, in the so-called "bedroom view".
Plant protection product - herbicide
| Structural formula of the pyridinium compound | Application example |
|---|---|

Field after herbicide exposure
|
The highly toxic paraquat dichloride is used as an effective herbicide.
Brominating agents
Pyridinium perbromide is an excellent brominating agent and has several advantages over elemental bromine, such as very precise weighing in small-scale reactions. An example of its application is the bromination of the 3-keto steroid 1 to form 2,4-dibromocholestanone ( 2 ):
Oxidizing agent
Pyridinium chlorochromate (PCC) can be used as a strong oxidizing agent in organic chemistry, for example for the oxidation of a primary alcohol to an aldehyde or analogously for the oxidation of a secondary alcohol to a ketone . It should be noted that PCC is only used in the laboratory due to its poor atom economy and the harmful effects of chromium compounds. The figure shows the example of the oxidation of the natural substance citronellol ( 1 ) to form citronellal ( 2 ), which also occurs in nature :
Individual evidence
- ↑ a b c d e f g h Jürgen Falbe, Manfred Regitz: Römpp-Chemie-Lexikon . Thieme, Stuttgart 1992, ISBN 3-13-735009-3 , p. 3696-3697 .
- ↑ a b c Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 802-804 .
- ↑ Jie Jack Lie: Name reactions: a collection of detailed reaction mechanisms . Springer-Verlag Berlin Heidelberg, Berlin, Heidelberg 2006, ISBN 978-3-540-30031-1 , p. 637-639 , doi : 10.1007 / 3-540-30031-7_288 .
- ↑ Red List , as of 2013.
- ↑ Carl Djerassi, Caesar R. Scholz: Brominations with Pyridine Hydrobromide Perbromide . In: Journal of the American Chemical Society . 70, No. 1, January 1948, pp. 417-418. doi : 10.1021 / ja01181a508 .
- ^ EJ Corey, J. William Suggs: Pyridinium Chlorochromate. An Efficient Reagent for Oxidation of Primary and Secondary Alcohols to Carbonyl Compounds . In: Tetrahedron Letters . 16, No. 31, June 1975, pp. 2647-2650. doi : 10.1016 / S0040-4039 (00) 75204-X .