Pyridinium tribromide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Pyridinium tribromide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 6 Br 3 N | |||||||||||||||
| Brief description |
red odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 319.82 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
130 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Pyridinium tribromide is a chemical compound of bromine from the group of pyridinium compounds .
Extraction and presentation
Pyridinium tribromide can be obtained by reacting pyridinium hydrobromide with bromine or thionyl bromide .
properties
Pyridinium tribromide is a crystalline red odorless solid that is practically insoluble in water.
use
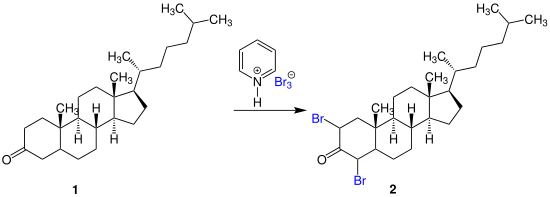
Pyridinium tribromide is used as a bromination reagent for ketones , phenols and ethers . It has several advantages over elemental bromine, such as very precise weighing in small-scale reactions. An example of its application is the bromination of the 3-keto steroid 1 to form 2,4-dibromocholestanone ( 2 ):
Individual evidence
- ↑ a b Data sheet pyridinium bromide perbromide (PDF) from Merck , accessed on August 13, 2017.
- ↑ a b c d data sheet Pyridinium tribromide, technical grade, 90% from Sigma-Aldrich , accessed on August 13, 2017 ( PDF ).
- ↑ a b c d data sheet Pyridine hydrobromide perbromide, tech. 90% at AlfaAesar, accessed August 13, 2017 ( PDF )(JavaScript required) .
- ^ Houben-Weyl Methods of Organic Chemistry Vol. V / 4, 4th Edition: Bromine and Iodine Compounds . Georg Thieme Verlag, 2014, ISBN 978-3-13-180014-5 , p. 35 ( books.google.de ).
- ↑ Jürgen Falbe, Manfred Regitz: Römpp-Chemie-Lexikon . Thieme, Stuttgart 1992, ISBN 3-13-735009-3 , p. 3696-3697 .
- ↑ Carl Djerassi, Caesar R. Scholz: Brominations with Pyridine Hydrobromide Perbromide . In: Journal of the American Chemical Society . 70, No. 1, January 1948, pp. 417-418. doi : 10.1021 / ja01181a508 .
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer Spectrum, Berlin Heidelberg 2013, ISBN 978-3-642-34715-3 , p. 802-804 .