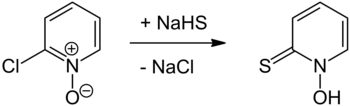Pyrithione
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Pyrithione | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 5 H 5 NOS | |||||||||
| Brief description |
beige-colored to gray-black solid with a foul odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 127.18 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| boiling point |
69-72 ° C |
|||||||||
| solubility |
sparingly soluble in water (2.5 g l −1 at 20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Pyrithione is an organic compound . It consists of a pyridine ring , which is oxidized on the nitrogen atom ( pyridine- N -oxide ) and has a thiocarbonyl function in the 2-position . It is a fungicide and a bactericide .
presentation
For the preparation of the compound is first 2-chloropyridine N -oxide with sodium hydrogen sulfide to the reaction brought, from which the sodium salt of pyrithione is formed. Pyrithione can be released from this by neutralization with acids .
properties
Pyrithione is a foul-smelling solid that melts at 69–72 ° C. It has only a low solubility in water of 2.5 g · l −1 at 20 ° C.
There is an equilibrium between the tautomeric enthiol and thione forms ( thiolactam-thiolactim tautomerism ).
use
Pyrithione can be used to make zinc pyrithione , which is used against dander and in anti-fungal paints. Sodium pyrithione , used as an antifungal agent , can be made by reacting with sodium salts . The bactericide and fungicide dipyrithione is produced by oxidative dimerization .
Pyrithione can also be used to produce copolymers of cellulose . This polymerization takes place radically .
In chemistry, pyrithione is used to produce Barton esters , which are required for Barton-McCombie decarboxylation . Derivatized with 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NDB-Cl) serves pyrithione in chemical analysis to UV - fluorescence -type detection in HPLC measurements.
Individual evidence
- ↑ a b c data sheet Pyrithione at AlfaAesar, accessed on June 6, 2010 ( PDF )(JavaScript required) .
- ↑ a b sheet pyrithione from Acros, accessed on 6 June of 2010.
- ↑ a b c d e f g h Entry on pyrithione. In: Römpp Online . Georg Thieme Verlag, accessed on June 10, 2014.
- ↑ a b Data sheet 2-Mercaptopyridine N-oxide from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ^ CJ Chandler, IH Segel: "Mechanism of the antimicrobial action of pyrithione: effects on membrane transport, ATP levels, and protein synthesis", in: Antimicrob. Agents Chemother. , 1978 , 14 , pp. 60-68; PMC 352405 (free full text, PDF).