Dipyrithione
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
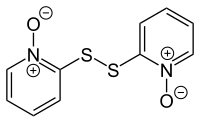
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dipyrithione | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 8 N 2 O 2 S 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 252.31 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
205 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dipyrithione is a chemical compound from the group of pyridines and disulfides and a derivative of pyrithione .
Extraction and presentation
Dipyrithione can be obtained from 2-chloropyridine . This reacts with peracetic acid to form 2-chloropyridine- N -oxide and further with sodium hydrogen sulfide (NaSH) to form 2-mercaptopyridine- N -oxide , which dimerizes through reaction with chlorine and sodium hydroxide .
use
Dipyrithione is fungicidal and bactericidal and is used in photographic materials, textile processing and agriculture.
Admission
Dipyrithione is not approved as a crop protection agent in the European Union or Switzerland .
toxicology
In an experiment with mouse cells, dipyrithione led to apoptosis of cancer cells.
Individual evidence
- ↑ a b entry on pyrithione. In: Römpp Online . Georg Thieme Verlag, accessed on June 10, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 853 ( preview ).
- ↑ Huang Huang, Yu Pan, Yin Ye, Min Gao, Zhimin Yin, Lan Luo: Dipyrithione attenuates oleic acid-induced acute lung injury . In: Pulmonary Pharmacology & Therapeutics . tape 24 , 2011, p. 74-80 , doi : 10.1016 / j.pupt.2010.09.008 ( PDF ).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national directory of plant protection products in Switzerland ; Retrieved June 25, 2016.
- ↑ Yumei Fan, Caizhi Liu, Yongmao Huang, Jie Zhang, Linlin Cai, Shengnan Wang, Yongze Zhang, Xianglin Duan, Zhimin Yin: Dipyrithione induces cell-cycle arrest and apoptosis in four cancer cell lines in vitro and inhibits tumor growth in a mouse model . In: BMC Pharmacology and Toxicology . tape 14 , no. 1 , 2013, p. 54 , doi : 10.1186 / 2050-6511-14-54 ( PDF ).