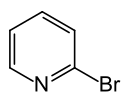
2-bromopyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-bromopyridine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 4 BrN | |||||||||||||||
| Brief description |
colorless to yellowish liquid with a pyridine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 158.00 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.62 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−40.1 ° C |
|||||||||||||||
| boiling point |
193 ° C |
|||||||||||||||
| Vapor pressure |
1.02 h Pa (25 ° C) |
|||||||||||||||
| solubility |
moderate in water (20 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.5734 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Bromopyridine is an organic compound that belongs to the heterocycles (more precisely: heteroaromatic compounds ). It consists of a pyridine ring, which is substituted in the 2-position with bromine .
presentation
In general, common electrophilic aromatic substitutions on pyridine are not possible or only possible with poor yield. Furthermore, pyridine would preferentially be substituted in the 3-position and 2-bromopyridine would only arise as a by-product. However, 2-bromopyridine can be prepared by reacting with molecular bromine in the presence of catalytic amounts of palladium (II) chloride .
use
2-bromopyridine and 2-chloropyridine can be converted to 2,2'-bipyridine in a Ullmann coupling , for which copper dust is used . It can also be used for the synthesis of 2,4'-bipyridine . To this end , it can be reacted with 4-bromopyridine in a Negishi coupling . For this purpose, 2-bromopyridine is first lithiated with n- butyllithium and transmetallated to zinc organyl with the addition of zinc chloride . A palladium complex with triphenylphosphine ligands is used as the coupling catalyst .
By lithiation , 2-bromopyridine can be converted into an organolithium compound , which serves as a starting compound for other pyridine derivatives that are not directly accessible.
Individual evidence
- ↑ a b c d e f g Data sheet 2-bromopyridine (PDF) from Merck , accessed on March 20, 2011.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-70.
- ^ A b J. A. Joules, K. Mills: Heterocyclic Chemistry , 2000 , 4th edition, Blackwell Science, Oxford, ISBN 0-632-05453-0 , pp. 77-81.
- ^ FH Burstall: Researches on the polypyridyls , in: J. Chem. Soc. , 1938 , pp. 1662-1671; doi : 10.1039 / JR9380001662 .
- ^ DR Sidler, N. Barta, W. Li, E. Hu, L. Matty, N. Ikemoto, JS Campbell, M. Chartrain, K. Gbewonyo, R. Boyd, EG Corley, RG Ball, RD Larsen, PJ Reider , Paul J: Efficient synthesis of the optically active dihydropyrimidinone of a potent α 1A -selective adrenoceptor antagonist. In: Canadian Journal of Chemistry . 80 (6), 2002, pp. 646-652, doi : 10.1139 / v02-079 .

