Butyric acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
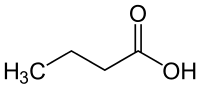
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Butyric acid | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 4 H 8 O 2 | |||||||||||||||||||||
| Brief description |
colorless, unpleasant smelling liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 88.11 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.9528 g cm −3 (25 ° C) |
|||||||||||||||||||||
| Melting point |
-5-6 ° C |
|||||||||||||||||||||
| boiling point |
163.7 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| pK s value |
4.82 |
|||||||||||||||||||||
| solubility |
miscible with water, ethanol and diethyl ether |
|||||||||||||||||||||
| Refractive index |
1.3980 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Butyric acid is the common name of butanoic acid , a carboxylic acid and at the same time the simplest fatty acid . It occurs naturally through butyric acid fermentation . Their vapors irritate the eyes and the respiratory tract. The salts (see below) and esters (see butyric acid esters ) of butyric acid are called butyrates (systematically also butanoates ). Its isomer is isobutyric acid .
history
Butyric acid was discovered in 1814 by Eugène Chevreul among the saponification products of butter and was carefully described. The origin of the butter fat and the smell of butter (Latin butyrum ) led to the name of the acid . Théophile-Jules Pelouze described them and their reactions in more detail in 1843, naming the following properties:
“Butyric acid is a completely colorless liquid , transparent, highly mobile, it has a smell that is reminiscent of acetic acid and strong butter . It is soluble in all proportions, in water, alcohol and wood spirit . At ordinary temperature it boils at 164 ° C and distills without any noticeable change. Its vapor is flammable and burns with a blue flame [...] its taste is strongly sour and burning. It attacks the skin and destroys it like the strongest acids. "
properties
Butyric acid essentially makes up the unpleasant smell of vomit or rancid butter. Butyric acid also contributes to sweat odor and, in some cases, bad breath . The vapors irritate the eyes and the respiratory tract. Butyric acid is produced when butter turns rancid. It dissolves very well in water and also in ethanol , diethyl ether and glycerine . Butyric acid is a weaker acid compared to formic acid and acetic acid . Iron , zinc , magnesium and other base metals dissolve very slowly with the evolution of hydrogen . Here, butyrates, in the form of moisture smell also of butyric acid:
- Reaction of magnesium with butyric acid
With alcohols may esters are produced. For this reaction is heat and a catalyst such. B. Sulfuric acid necessary:
- Reaction of butyric acid with ethanol
Butyric acid forms flammable vapor-air mixtures at high temperatures. The compound has a flash point of 72 ° C. The explosion range is between 2.0% by volume (72 g / m³) as the lower explosion limit (LEL) and 10.0% by volume (365 g / m³) as the upper explosion limit (UEL). A correlation of the explosion limits with the vapor pressure function results in a lower explosion point of 64 ° C. The ignition temperature is 440 ° C. The substance therefore falls into temperature class T2.
Occurrence
Since butyric acid is formed from carbohydrates by butyric acid bacteria under anaerobic conditions , it occurs in foods that require fermentation processes for their preparation , e.g. B. Cheese , sauerkraut , beer and bread; it is also found in milk, meat juice and sweat , as well as in wood vinegar . It is also found in some plant lipids, mostly in low concentrations . The original assumption that the foul-smelling, pungent and corrosive liquid that various species of ground beetles (Carabidae) such as the real ground beetles ( Carabus spp.) Spray as defense from the pygiadial gland contains butyric acid, was put into perspective in later studies.
Manufacturing
The gram-positive , anaerobic , spore-forming bacterium Clostridium tyrobutyricum is able to produce butyric acid through fermentation . It has the ability to break down both glucose and xylose . The main metabolic end products are butyric acid, acetic acid , hydrogen and carbon dioxide . The simplified reaction equation is:
However, the yield in fermentations is considerably lower than the theoretical maximum, since butyric acid production is accompanied by acetic acid production . The production of fermentative butyric acid is mainly carried out in synthetic growth media with glucose , xylose or sucrose as the carbon source. During the past decade, when the concept of sustainable production of fuels and chemicals from residual raw materials was paramount, butyric acid was made from corn fiber. Using lignocellulose for the biological production of fuels and chemicals requires pre-treatment and enzymatic hydrolysis to release glucose and xylose from the lignocellulose matrix. Depending on the raw material and the hardness of the pretreatment, pretreatment processes also release toxic compounds such as carboxylic acids , furan derivatives and phenolic compounds that inhibit microbial metabolism and growth . Therefore, inhibition could be one of the first obstacles to be overcome when using hydrolysates from second generation biomass for biological production processes, especially when using undiluted hydrolysates with high sugar concentrations.
Metabolism in the gut
In the human large intestine, butyric acid is mainly produced when prebiotic carbohydrates are broken down by intestinal bacteria. The associated pH value shift into the acidic range makes the environment unfavorable for salmonella and other pathogens. In addition, butyric acid appears to directly stimulate bowel movements and serves as an energy source for the epithelial cells of the colon.
odor
Small traces of the smell of butyric acid can be perceived by humans and animals. Concentrations from 0.06 mg per cubic meter can be perceived by humans. Humans rate the smell negatively, the housefly, however, positively. Ticks use the smell of butyric acid to find their hosts.
Along with propionic acid , hydrogen sulfide and volatile organic compounds containing sulfur ( methanethiol , dimethyl sulfide ), butyric acid is a cause of bad breath in humans.
Since the formation of butyric acid is a sign of putrefaction , its odor perception serves as a warning odor. The odor of butyric acid can be reduced with bases such as caustic soda , solutions of carbonates , etc. In the process, odorless butyrates are formed.
use
In addition to hydrogen sulfide, butyric acid is used to produce inexpensive, particularly effective and long-lasting stink bombs . The penetrating odorous substance is also used to drive away animals with a fine sense of smell, such as moles.
Butyric acid is used in various industries . There is currently great interest in using it as a precursor for biofuels , e.g. B. biobutanol to use. With the rise in the price of oil , the continued decline in the availability of petroleum and the growing need for clean energy sources , research has recently been directed towards alternative fuel sources .
Butyric acid also has numerous uses in the pharmaceutical and chemical industries . First, butyric acid for their anti-cancer activity known since it in a variety of cells , a morphological and biochemical differentiation induced, which for the simultaneous suppression of the neoplastic leads properties. As a result, these studies of various prodrugs that are derivatives of butyric acid were examined for their potential use in the treatment of cancer and hemoglobinopathies , including leukemia and sickle cell anemia , and in protecting hair follicles from radiation and chemotherapy- induced diseases.
In the chemical industry butyric acid is mainly used for the production of cellulose - plastics used. Butyric acid esters are used as flavorings and fragrances in the beverage, food and cosmetics industries.
Salts
Butyrate (systematically also butanoate ) is not only a name for butyric acid ester but also the name for the salts of butyric acid. These consist of butyrate anions C 3 H 7 COO - and a cation . Examples are sodium butyrate (NaC 3 H 7 COO), magnesium butyrate [Mg (C 3 H 7 COO) 2 ] and ammonium butyrate ( NH 4 C 3 H 7 COO). When wet, they have the same characteristic odor as butyric acid. If a butyrate salt is treated with a stronger acid , butyric acid is produced again.
Ester
The esters of butyric acid have a fruit odor in many cases and occur naturally in many fruit aromas.
Web links
Individual evidence
- ↑ entry to butyric acid in the CosIng database of the European Commission, accessed on February 26 2020th
- ↑ a b c d e f g h i j k l m n Entry on butyric acid in the GESTIS substance database of the IFA , accessed on May 6, 2020(JavaScript required) .
- ↑ a b Entry on butyric acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 96th edition. (Internet version :), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-78.
- ^ A b Gerhard Eisenbrand, Peter Schreier: RÖMPP Lexikon Lebensmittelchemie. 2nd edition, Thieme, 2006 ISBN 978-3-13-736602-7 , p. 160.
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , p. 1118.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-74.
- ↑ Entry on Butyric acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c Théophile-Jules Pelouze , Amédée Gélis: About butyric acid . In: Friedrich Wöhler, Justus Liebig (Ed.): (Justus Liebigs) Annals of Chemistry and Pharmacy . tape 47 , no. 3 . CF Winter, Heidelberg 1843, p. 241–253 , doi : 10.1002 / jlac.18430470302 ( limited preview in Google Book search).
- ↑ Théophile-Jules Pelouze, Amédée Gélis: About butyric acid . In: Otto Linné Erdmann, Richard Felix Marchand (Hrsg.): Journal for practical chemistry . tape 29 , no. 1 . Johann Ambrosius Barth, Leipzig 1843, doi : 10.1002 / prac.18430290171 ( bsb-muenchen.de ).
- ↑ Théophile-Jules Pelouze, Amédée Gélis: Mémoire sur l'acide butyrique . In: J.-B. Dumas (Ed.): Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences . tape 16 , no. 1 . Bachelier, Paris January 2, 1843, pages 1268–1269 Éther butyrique, p. 1262–1271 ( online at Gallica Bibliothèque numérique ).
- ↑ Thomas Seilnacht: Butyric acid
- ↑ J. Schormüller : The components of food. Springer, 1965, ISBN 978-3-642-46012-8 , pp. 765-766.
- ↑ H. Schildknecht, H. Winkler. U. Maschitz: Comparative chemical investigations of the constituents of the pygidial defense bladders of carabids. In: Z. Naturforsch. 23 b, 1968, pp. 637-644, doi : 10.1515 / znb-1968-0512 (PDF; 6.9 MB).
- ↑ GN Baroi, I. Baumann, P. Westermann, HN Gavala: Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted Clostridium tyrobutyricum strain. In: Microbial biotechnology. Volume 8, number 5, September 2015, pp. 874-882, doi : 10.1111 / 1751-7915.12304 , PMID 26230610 , PMC 4554475 (free full text).
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance. Vieweg + Teubner Verlag, 2011, ISBN 978-3-8348-1245-2 , pp. 61-62.
- ↑ George A Burdock: Fenaroli's Handbook of Flavor Ingredients. Sixth Edition, CRC Press, 2009, ISBN 978-1-4200-9077-2 .






