Sodium butyrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium butyrate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 7 NaO 2 | |||||||||||||||
| Brief description |
hygroscopic white powder with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 110.09 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.96 g cm −3 |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium butyrate is a white, hygroscopic sodium salt of butyric acid . It has an unpleasant odor and is stable to light and heat.
synthesis
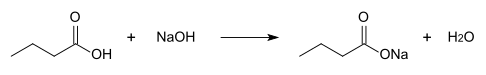
Sodium butyrate can be produced from sodium hydroxide and butyric acid by the salt formation reaction .
use
Sodium butyrate is used as an additive in animal feed. It is used in medicine as a nutrient for intestinal cells and it accelerates the regeneration of the intestinal lining . It also influences the growth of colon cancer cells and is marketed under the name Sobutir ® . Research has shown that sodium butyrate may be useful in the treatment of spinal muscular atrophy . For the prophylaxis of radiogenic proctitis it was registered by the European Commission under the number EU / 3/05/284 in the Community Register for Orphan Medicinal Products.
Individual evidence
- ↑ a b c d e f Data sheet sodium butyrate (PDF) from Merck , accessed on May 19, 2010.
- ↑ a b Entry at www.chemischemlexikon.de , accessed on May 19, 2010.
- ↑ Sodium butyrate data sheet from AlfaAesar, accessed on May 19, 2010 ( PDF )(JavaScript required) .
- ↑ Sodium butyrate data sheet from Sigma-Aldrich , accessed on June 13, 2011 ( PDF ).
- ↑ a b Entry for CAS no. 156-54-7 in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Olof Dietz, Bernhard Huskamp (ed.): Handbuch Pferdepraxis . Georg Thieme Verlag, 2006, ISBN 3-8304-1028-X , p. 443 ( limited preview in Google Book search).
- ↑ Aldo Roda, Patrizia Simoni, Maria Magliulo, Paolo Nanni, Mario Baraldini, Giulia Roda, Enrico Roda: A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon . In: World Journal of Gastroenterology . tape 13 , no. 7 , February 21, 2007, p. 1079-1084 , doi : 10.3748 / wjg.v13.i7.1079 , PMC 4146871 (free full text).
- ↑ Instructions for use Sobutir. (PDF; 210 kB) (No longer available online.) In: juvalis.de. Archived from the original on April 12, 2016 ; Retrieved April 12, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ JG Chang, HM Hsieh-Li, YJ Jong, NM Wang, CH Tsai, H. Li: Treatment of spinal muscular atrophy by sodium butyrate. In: Proc Natl Acad Sci USA. 98 (17), Aug 14, 2001, pp. 9808-9813. PMID 11504946 PMC 55534 (free full text).
- ↑ EMA: PUBLIC SUMMARY OF POSITIVE OPINION FOR ORPHAN DESIGNATION OF sodium butyrate (rectal use) for the prevention of radiation proctitis (PDF; 53 kB), June 29, 2005.