Sulfinic acids
| Sulfinic acid and sulfinates |
|---|
 Sulfinic acid |
 Sulfinates |
| R is an organyl group . The functional group is marked in blue . |
Sulfinic acids are a class of chemical compounds with organically bound sulfur and oxygen with the general structure R – S (= O) –OH, where R is an alkyl or aryl radical . The basic substance sulfinic acid has the structure H – S (= O) –OH and is tautomeric to sulfoxylic acid . The salts of sulfinic acids are the sulfinates .
presentation
Acids
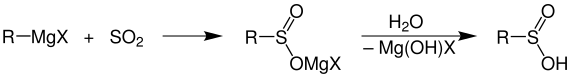
Sulphinic acids, which occur as an intermediate stage in the oxidation of thiols , cannot be isolated. Sulfinic acids are accessible through
Grignard reaction , in which an organometallic reagent reacts with sulfur dioxide
or by reduction of sulfonyl chlorides by means of zinc dust.
Ester
Sulfinic acid esters can be easily prepared by reacting disulfides with N-bromosuccinimide in the presence of the ester-forming alcohol. The reaction with primary alcohols is good, with secondary alcohols a small addition of potassium carbonate has a beneficial effect. Dichloromethane can serve as a cosolvent. Alcohols that react directly with the halogenating agent are unsuitable for this method. The required symmetrical disulfides can be conveniently prepared by oxidative coupling of thiols in an air atmosphere.
properties
The low-molecular representatives are viscous oils, the higher homologues are crystalline substances, and most are readily soluble in water. Many sulfinic acids are unstable. All sulfinic acids are oxidized to sulfonic acids by oxygen in the air . Even in the absence of air, they gradually disproportionate .
The sulfinic acids are moderately strong acids (p K S ≈ 2-3) and form stable salts, the sulfinic acid salts. Other stable derivatives are, for example, the sultines , which are to be understood as cyclic esters .
Reactions
Acids
Treatment with reducing agents such as nascent hydrogen - e.g. from zinc and hydrochloric acid - leads to thiols :
The sulfinic acid salts react with haloalkanes to form sulfones :
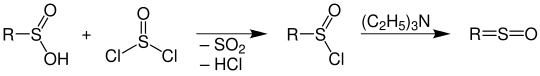
In the reaction with thionyl chloride , sulfinic acids are converted into sulfinic acid halides ( sulfinyl halides ); these very reactive substances can - e.g. Example by the action of triethylamine - to ketenes analog sulfines (according to IUPAC Thioaldehydoxide and Thioketonoxide ) are reacted:
Ester
Reactive aromatics can be sulfinylated using the Friedel-Crafts reaction. Sulfinic acid esters are suitable to replace the more sensitive sulfinyl chlorides in the Friedel-Crafts sulfinylation . The reaction takes place under mild conditions.
See also
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 591.
- ^ A b c Siegfried Hauptmann: Organic Chemistry . German publishing house for basic industry, 1985, ISBN 3-87144-902-4 , p. 480-482 .
- ↑ Peter Brown Bridge, Ian C. Jowett: 'One-pot' synthesis of Sulphinic ester from Disulphides . In: Synthesis . 1988, No. 03, 1988, pp. 252-254. doi : 10.1055 / s-1988-27535 .
- ↑ José Luis García Ruano, Alejandro Parra, José Alemán: Efficient synthesis of disulfides by air oxidation of thiols under sonication . In: Green Chemistry . 10, No. 6, 2008, p. 706. doi : 10.1039 / b800705e .
- ↑ Entry on sulfinic acids. In: Römpp Online . Georg Thieme Verlag, accessed on February 17, 2013.
- ↑ a b Wissenschaft-Online-Lexika: Entry on "Sulphinic Acids" in the Lexikon der Chemie , accessed on February 17, 2013.
- ↑ Hans Beyer: Textbook of organic chemistry . S. Hirzer Verlag, 1998, ISBN 3-7776-0808-4 , p. 158-160 .
- ^ Francisco Yuste, Angélica Hernández Linares, Virginia M. Mastranzo, Benjamín Ortiz, Rubén Sánchez-Obregón, Alberto Fraile, José Luis García Ruano: Methyl Sulfinates as Electrophiles in Friedel – Crafts Reactions. Synthesis of Aryl Sulfoxides . In: The Journal of Organic Chemistry . 76, No. 11, 2011, pp. 4635-4644. doi : 10.1021 / jo2006335 .