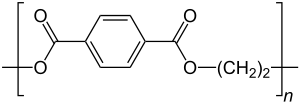
Polyethylene terephthalate
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Polyethylene terephthalate | ||||||
| other names |
|
||||||
| CAS number | 25038-59-9 | ||||||
| Monomers | Ethylene glycol and terephthalic acid | ||||||
| Molecular formula of the repeating unit | C 10 H 8 O 4 | ||||||
| Molar mass of the repeating unit | 192.17 g mol −1 | ||||||
| Type of polymer | |||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.38 g cm −3 (20 ° C) |
||||||
| Melting point |
250-260 ° C |
||||||
| Glass temperature |
70 ° C |
||||||
| Crystallinity |
partially crystalline |
||||||
| modulus of elasticity |
4500 N mm −2 lengthways and crossways |
||||||
| Water absorption |
0.5% |
||||||
| solubility |
practically insoluble in water |
||||||
| Thermal expansion coefficient |
7 · 10 −5 K −1 |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polyethylene terephthalate ( abbreviated PET ) is a by polycondensation made of thermoplastic plastic material from the family of polyesters . PET has a wide range of applications and is used, among other things, to manufacture plastic bottles ( PET bottles ), foils and textile fibers. In 2008 the production was 40 million tons. Despite increased recent recycling, the production volume increased to 56 million tons by 2016.
history
Polyethylene terephthalate goes back to an invention of the two Englishmen John Rex Whinfield and J. T. Dickson. In 1941, in the laboratories of the Calico Printers Association textile company in Accrington , they succeeded for the first time in producing a polyester from ethylene glycol and terephthalic acid and in making fibers from it . GB Patent No. 578 079 describes this invention. Since it fell during the Second World War, the description of the invention as well as a successor patent were declared secret patents. Since further development work could not be carried out at the Calico Printers Association, this was taken over by a British government institute. Because of the limited possibilities there, the British chemical company ICI was commissioned with further research and development work at the end of 1943 . In addition, the British government pushed for a contractual agreement between Calico Printers and ICI for the latter to take over the manufacturing rights. The ICI received this in 1947 worldwide, with the exception of the USA. Two years later, the ICI began the trial production of fibers from polyethylene terephthalate under the brand name "Terylene". Through the cooperation of the ICI with the American chemical company Du Pont , information about the invention reached this company, where its own development work was carried out from 1944 onwards. Du Pont acquired the manufacturing rights for the USA and in 1949 began trial production of a fiber that was later given the brand name " Dacron ". Large-scale fiber production began in 1953 at both ICI and Du Pont. By granting licenses, fibers from polyethylene terephthalate could also be manufactured in Germany and brought onto the market under the brand names “Trevira” and “Diolen”. In the 1970s in particular, there was a strong expansion in the production of these fibers worldwide. Their rapid economic success can be seen as unique in the textile world. Polyethylene terephthalate fibers hold the leading position among synthetic fibers. The main manufacturing country today is the People's Republic of China.
properties
Physical Properties
PET is polar , which means that there are strong intermolecular forces. The molecule is also built up linearly without cross-links. Both are prerequisites for partially crystalline areas and fibers. These areas also result in high breaking strength and dimensional stability at temperatures above 80 ° C. The impact strength is low, however, and the sliding and wear behavior is good. The glass transition temperature is around 80 ° C. PET changes into the partially crystalline state (C-PET) at around 140 ° C. The unit cell is triclinic {a = 4.56 nm, b = 5.94 nm, c = 10.75 nm, α = 98.5 °, β = 118 °, γ = 112 °}. The density of amorphous PET (A-PET) is 1.33-1.35 g · cm³ and of partially crystalline C-PET 1.38-1.40 g · cm³. Compared to C-PET, A-PET has a slightly lower stiffness and hardness, but higher impact strength. The density of the crystalline areas depends on the duration and the temperature of the solid phase polycondensation , which is carried out as standard to achieve higher molecular weight PET grades. The degree of crystallization hardly exceeds 70%. The melting point is (depending on the degree of crystallization and the degree of polymerization ) between 235 and 260 ° C.
When heated up strongly, a bottle made of PET by blowing will partially contract again - similar to vacuum-drawn yoghurt pots made of PS .
Chemical properties
Polyethylene terephthalates are resistant to many chemicals and are therefore preferred as containers for liquids in the food industry , but also in laboratories and medicine .
However , PET is not resistant to strong inorganic acids , especially sulfuric acid or nitric and hydrochloric acid .
The analysis of PET by means of 1 H and 13 C NMR spectroscopy is described in the literature.
Manufacturing
The monomers from which PET is made are terephthalic acid (1,4-benzene dicarboxylic acid) and ethylene glycol (1,2-dihydroxyethane, ethane-1,2-diol, ethanediol).
Large-scale production is still partly carried out by transesterification of dimethyl terephthalate with ethanediol. Since this is an equilibrium reaction, an excess of ethanediol is used, which is distilled off again as the reaction proceeds in order to favorably influence the equilibrium. The melt phase polycondensation does not lead (in technically reasonable periods of time) to sufficiently high molecular weights. This is why PET grades for bottles or industrial yarn (e.g. Diolen, Trevira) are subsequently condensed further using solid-state polycondensation (SSP).
Ring opening reactions from oligomers are also possible, whereby no condensate is produced and high molar masses of more than 100,000 g / mol can be achieved quickly. However, these methods are still under development.
In more recent processes, ethanediol is esterified directly with terephthalic acid . For processing, PET in partially crystalline form is preferred, but it only crystallizes very slowly spontaneously, which is why nucleating agents must be added for rapid crystallization.
PET often contains traces of antimony (III) oxide , which is used as a catalyst during manufacture.
raw materials
Partially bio-based PET, in which the ethylene glycol component (approx. 30% of the product) is made from renewable raw materials, has been on the market since around 2010. In 2017, around 1.09 million tons of it were produced, making it the largest share of all (partially) bio-based plastics. In mid-2015, a completely bio-based PET bottle was presented for the first time at the world exhibition in Milan. The completely biogenic product is currently not ready for the market, as no bio-based production for terephthalic acid has yet become established.
use
PET is processed in many forms and used in a variety of ways. The most well-known uses include the production of plastic bottles ( PET bottle , injection blow molding , stretch blow molding ) of all kinds and processing into textile fibers . PET is also used to produce film material such as that used in the cinema. PET has been used to manufacture very thin films since the 1950s, often under the name Hostaphan ® , Mylar ® .
PET is used as a textile fiber ( polyester ) because of its various useful properties. It is wrinkle-free, tear-proof, weather-resistant and absorbs very little water. The latter predestines PET as a material for sportswear that has to dry quickly.
PET is also preferred in the food industry. It can be processed amorphously and in this form is absolutely colorless and has a high level of light permeability. It is used for food packaging and bottles such as B. the PET bottle . Uncoated PET has insufficient gas tightness , which is why a diffusion barrier, usually made of silicon dioxide , is applied to sensitive drinks or food such as fruit juices, beer, wine or ketchup . This layer is applied in the plasma process either inside or outside the plasma.
However, acetaldehyde is also produced during the production of PET bottles , which can migrate into the content in small quantities (also in the case of mineral waters) and change the taste (sensory). Antimony (III) oxide (antimony trioxide), which is often used in production, can also dissolve in the liquid content of a PET bottle. Investigations of fruit juices filled in PET bottles showed antimony trioxide concentrations of <1 up to 44.7 µg / L in undiluted juice concentrates. The applicable limit value (so-called specific migration limit) for the transfer of antimony trioxide from plastic to food is 40 µg / L. This value was partially exceeded in ready-made baked goods, especially because antimony trioxide can be more easily dissolved at high temperatures.
Antimony trioxide is listed under number 051-005-00-X in the annex of Regulation (EC) No. 1272/2008 . The classification given there was carried over from the previously valid regulation 67/548 / EEC . In the old regulation, consideration of the route of delivery for the endpoint carcinogenicity was not provided, whereas the current regulation provides for the specification of the route of delivery if there is sufficient data; Antimony trioxide is classified as a carcinogen in hazard category 2 (H 351; suspected of causing cancer if inhaled). The International Agency for Research on Cancer (IARC) considers antimony trioxide a "Potentially Carcinogenic Substance" (IARC Category 2B). The classification was made on the basis of three chronic inhalation studies in rats. The most likely mechanism of carcinogenicity is due to impaired lung cleaning after particle overload, the an inflammatory reaction, fibrosis and tumor formation follow. Antimony trioxide can therefore be regarded as a carcinogen with a threshold value. In this context, however, it is questionable whether the effects of lung overload in rats are also relevant for humans (European Commission, European Union Risk Assessment Report Diantimony trioxide, 2008) There is no suspicion of a carcinogenic effect after oral administration.
Because of its good tissue compatibility, PET is also used as a material for vascular prostheses .
Polyester film
A large and important area of application for polyethylene terephthalate (PET) are films , which today are produced in thicknesses of 1 to 800 µm. They are used for thin films for plastic film capacitors , stamping films, packaging films for aroma-proof packaging, furniture films, colored light protection films , cine films , photo films, X-ray films , electrical insulation films , anchor groove insulation films , and films for test strips in the pharmaceutical industry. A large area of application are also carrier films for magnetic tapes. Typewriter tapes were also made from PET.
PET film is made from raw material granules that are first dried or directly from the melt (Uhde Inventa Fischer process). Drying prevents hydrolytic degradation during processing. A film is produced from it through a slot die by melting by means of extrusion and filtration. The liquid melt film is pressed onto a casting roller by means of electrostatic pinning and cooled below the glass transition temperature of PET, which is around 65 to 80 ° C. The electrostatic pinning is an application method for the liquid melt film, in which z. B. an uninsulated wire is attached parallel to the melt film at a millimeter distance. A high voltage of 5 to 10 kV is applied to the wire . Due to the dipole character of the PET molecules, the side of the film facing the wire is positively charged. The opposite pole is the earthed casting roller. With this process, the melt film is pressed against the casting roll, the air between the PET film and the cooled casting roll is displaced. This is important for the film to cool evenly and quickly. In this process step the so-called prefilm is created.
The film receives its final mechanical properties from the subsequent stretching process. The stretching usually takes place in two steps, first in the longitudinal direction and then in the transverse direction. For the stretching, the film has to be reheated to above the glass temperature. For longitudinal stretching, the film is passed over heated rollers, warmed up to the stretching temperature of z. B. 85 ° C heated and stretched in a stretching gap with an additional IR radiator in the longitudinal direction by 2.5 to 3.5 times. The rollers after the stretching nip rotate at a correspondingly higher speed.
The second step is spreading. This takes place in a spreader frame in which the film is held on the side edges by clip chains. Clip chains run parallel to the film web. Retaining clips are attached to the individual chain links to hold the edges of the film. First the film is warmed up with hot air and then stretched in width. The chains are guided on a guide rail in such a way that, after preheating, the distance between the clip chains is widened by 2.5 to 4 times. The last step is the thermal fixation of the film. The film still clamped in the clip chain is heated to a temperature between 200 and 230 ° C. This relieves tension in the film. Due to the heat setting, the molecular threads are so close together that a physical connection is created between the molecular chains due to the small distance. That is the crystallite formation.
In addition to this standard process, there are various variants to create special properties such as increased strength in the longitudinal direction.
If the film is to be used later at high temperatures (> 80 ° C) and remains dimensionally stable, it is advisable to pre-shrink this film in an oven above the later use temperature in order to make it tension-free. During this process, the dimensions of the film change according to the manufacturing process. Some manufacturers therefore also offer pre-shrunk films for high-temperature applications.
Depending on the desired application, pigments are also added to the raw material. This improves the winding properties of the finished film. Such films are also pigmented for matting furniture films. Colored pigments are also used. There are other, also soluble additives for UV stabilization and absorption or for coloring.
The raw material can also be modified by other polymer building blocks. If part of the terephthalic acid is replaced by isophthalic acid , the melting point of the raw material falls and the tendency to crystallize, resulting in PETIP . The linear chain formation is disturbed. By co-extrusion of PET with PETIP sealable films are prepared.
The finished foils are often coated or glued to form bonds with other foils. Associations are z. B. aroma-proof films for coffee packaging.
Metallized polyethylene terephthalate (MPET)
Metallized polyethylene terephthalate (abbreviation: MPET ) such as biaxially oriented PET ( boPET , Mylar ) is used for rescue blankets and was used as a flame retardant . Its inadequate fire resistance was identified as one of the causes of the serious accident on Swissair flight 111 on September 2, 1998.
recycling

Current procedures
PET has the recycling code 01. The high value of PET and the economic efficiency of recycling can also be seen in the fact that high-tech sorting processes have been developed for this using high-speed laser spectroscopy . They sort the fragments of shredded PET bottles , which in addition to the main component PET also contain other plastics (film layer in the wall, lid), according to type for recycling in food quality. The process was nominated for the German Future Prize in 2010. A ton of single-origin PET has a market price between 400 and 500 euros (as of 07/2014).
In 2009, 48.4% of all PET bottles across Europe were collected for recycling. 40% of the recycled PET was processed into textile fibers, 27% into foils and thermoformed products, 22% were used to manufacture new bottles and containers and 7% were used to manufacture plastic strapping .
research
More recent research opens up the prospect of degrading polyethylene terephthalate in future recycling processes through bacterial decomposition of plastic . According to research published in March 2016, polyethylene terephthalate is broken down into two substances ( terephthalic acid and ethylene glycol ) by the bacterium Ideonella sakaiensis 201-F6 , which could serve as the basis for better recycling.
Copolymers
PETG
PETG is a PET modified with glycol , which is characterized by its aqueous properties ( viscosity ). Applications can be found in injection molding and FDM 3D printing .
PEIT
PEIT (polyethylene-co-isosorbide terephthalate) is a copolymer in which isosorbide is used as a comonomer . The product has applications in the high temperature range and can be used for hot loaded containers and optical data storage devices .
Food safety
A statement in the journal Environmental Health Perspectives from April 2010 suggests that PET in the usual forms of use has hormone-active properties (so-called endocrine disruptors ). The article recommends further research into the material.
It is assumed that this property is due to the washing out of phthalates or antimony from the plastic. According to another article published in the Journal of Environmental Monitoring in April 2012, the concentration of antimony in demineralized water that is briefly stored in PET bottles at up to 60 ° C is within the European limit values, while Contents such as water or soft drinks that are stored in such bottles at room temperature for up to a year occasionally exceed these limit values.
One study measured the migration or elution of phthalic acid esters from polyethylene terephthalate bottles for soft drinks . The highest values contained drinks that were preserved with potassium sorbate or that had the lowest pH value . The measured values did not pose a health risk , but small amounts could accumulate over time.
Individual evidence
- ↑ a b c d Entry on polyethylene terephthalate in the GESTIS substance database of the IFA , accessed on July 30, 2017(JavaScript required) .
- ↑ a b Entry on polyethylene terephthalate. In: Römpp Online . Georg Thieme Verlag, accessed on January 16, 2014.
- ↑ a b c Mitsubishi Polyester Film GmbH, data comparison for plastic films (accessed on July 24, 2016).
- ^ Polyethylene terephthalate (PET). In: kunststoffe.de. Retrieved November 2, 2018 .
- ↑ Technical data sheet PET (PDF; 143 kB) from PolyQuick, accessed on April 2, 2013.
- ^ "Water instead of acid" , article by Deutschlandfunk from September 17, 2008 in the "Current Research" section.
- ↑ Shalini Saxena: Newly identified bacteria cleans up common plastic . In: Ars Technica . March 19, 2016 ( arstechnica.com ).
- ^ Römpp Lexikon Chemie, 9th edition 1992, page 3566
- ^ Kuhnke technologies: Chemical resistance of plastics , July 29, 2003, accessed on March 9, 2014.
- ↑ Antxon Martínez de Ilarduya, Sebastián Muñoz-Guerra: Chemical Structure and Microstructure of Poly (alkylene terephthalate) s, their Copolyesters, and their Blends as Studied by NMR . In: Macromolecular Chemistry and Physics . 215, No. 22, November 1, 2014, pp. 2138–2160. doi : 10.1002 / macp.201400239 .
- ↑ Sebastian Kotzenburg, Michael Maskus, Oskar Nuyken: Polymers - Synthesis, Properties and Applications , Springer Spectrum, 2014, pp. 195–196, ISBN 978-3-642-34772-6 .
- ↑ Michael Thielen: Bioplastics. Fachagentur nachwachsende Rohstoffe eV (FNR), 2019, accessed on September 20, 2019 .
- ↑ Institute for bioplastics and biocomposites: Biopolymers - Facts and statistics - capacities Production, processing routes, feed stock, land and water use. 2018, accessed September 20, 2019 .
- ↑ Christian Bonten: Plastics Technology : Introduction and Basics . 2nd updated edition. Hanse Verlag, 2016, ISBN 978-3-446-44917-6 , p. 475 .
- ↑ Julia Weiler: Plasma coating: how fewer gases get through the plastic layer - a touch of glass makes you leakproof. (No longer available online.) In: medizin-und-technik.de. February 12, 2015, archived from the original on August 30, 2016 ; accessed on August 30, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Internal plasma coating of PET bottles. In: hessen-nanotech.de. August 30, 2016, accessed August 30, 2016 .
- ↑ Keep plastic bottles tight: aif.de. In: aif.de. August 19, 2016. Retrieved August 30, 2016 .
- ↑ Mineral water test by Stiftung Warentest , test 8/2008, ISSN 0040-3946 .
- ↑ Claus Hansen, Alexandra Tsirigotaki, Søren Alex Bak, Spiros A. Pergantis, Stefan Stürup, Bente Gammelgaard, Helle Rüsz Hansen: Elevated antimony concentrations in commercial juices . In: Journal of Environmental Monitoring . tape 12 , no. 4 , 2010, p. 822-824 , doi : 10.1039 / B926551A .
- ↑ Directive 2002/72 / EC of the Commission on materials and objects made of plastic that are intended to come into contact with food in the consolidated version of November 9, 2009 , accessed on February 11, 2017 .
- ↑ FAA : Airworthiness Directive (English)
- ↑ unisensor.de: Products: PowerSort 200
- ^ German Future Prize: Laser light finds valuable materials - resources for our future ( Memento from September 14, 2013 in the Internet Archive ).
- ↑ January Garvert , who benefits from the recycling bin? ( Memento from July 14, 2014 in the Internet Archive ), tagesschau.de from July 11, 2014.
- ↑ KRONES magazine , 03/2012, p. 16.
- ↑ Garbage: Researchers discover plastic-eating bacteria. Spiegel online, March 11, 2016, accessed March 13, 2016 .
- ↑ A bacterium that degrades and assimilates poly (ethylene terephthalate). Science , March 11, 2016, accessed March 13, 2016 .
- ↑ Extrudr , Wieso PETG ( Memento of the original from September 25, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. As of Sept. 20, 2015.
- ↑ Daan S. van Es, Frits van der Klis, Rutger JI Knoop, Karin Molenveld, Lolke Sijtsma, and Jacco van Haveren: Other Polyesters from Biomass Derived Monomers . In: Stephan Kabasaci (Ed.): Bio-Based Plastics Materials and Applications . Wiley, 2014, ISBN 978-1-119-99400-8 , chap. 9 , p. 241-274 .
- ^ Leonard Sax: Polyethylene terephthalate may yield endocrine disruptors . In: Environmental Health Perspectives . tape 118 , no. 4 , April 2010, p. 445-448 , doi : 10.1289 / ehp.0901253 , PMID 20368129 , PMC 2854718 (free full text).
- ↑ Aminu Tukur, Liz Sharp, Ben Stern, Chedly Tizaoui, Hadj Benkreira: PET bottle use patterns and antimony migration into bottled water and soft drinks: the case of British and Nigerian bottles . In: Journal of Environmental Monitoring . tape 14 , no. 4 , April 1, 2012, doi : 10.1039 / c2em10917d .
- ↑ Jasna Bošnir, Dinko Puntarić, Antonija Galić, Ivo Škes, Tomislav Dijanić, Maja Klarić, Matijana Grgić, Mario Čurković, Zdenko Šmit: Migration of Phthalates from Plastic Containers into Soft Drinks and Mineral Water ; Food Technology and Biotechnology, vol. 45 no. 1, 2007 (abstract); (PDF file, full article )
literature
- JR Whinfield: The Development of Terylene . In: Textile Research Journal . tape 23 , no. 5 , 1953, pp. 289-293 , doi : 10.1177 / 004051755302300503 .
- Derek Moorhouse: Polyester: 50 years of achievement . In: Journal of the Society of Dyers and Colourists . tape 109 , no. 7-8 , 1993, pp. 255-255 , doi : 10.1111 / j.1478-4408.1993.tb01570.x .
- H. Vogler: The short but very successful history of polyester fibers. In: Chemical Fibers International. Volume 50, 2000, pp. 134-143.



