Isosorbide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Isosorbide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 10 O 4 | |||||||||||||||||||||
| Brief description |
White, odorless, crystalline, hygroscopic solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 146.14 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.30 g cm −3 |
|||||||||||||||||||||
| Melting point |
61-64 ° C |
|||||||||||||||||||||
| boiling point |
decomposes above 270 ° C |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
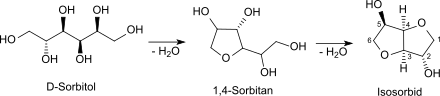
Isosorbide is a bicyclic chemical compound from the group of diols and oxygen-containing heterocycles in which two furan rings are fused . The starting material for isosorbide is D - sorbitol , which is obtained by catalytic hydrogenation of D- glucose , which in turn is produced from starch by hydrolysis . Isosorbide is therefore also being discussed as a plant-based platform chemical from which biodegradable derivatives of different functionality can be derived.
As a monomer component for biopolymer polycarbonates, polyesters, polyurethanes and epoxides, isosorbide is currently attracting great scientific and technical interest.
Extraction and presentation
Isosorbide is obtained from D -sorbitol by acid-catalyzed dimolecular dehydration , whereby the monocyclic furanoid sorbitan is initially formed, from which the bicyclic furofuran derivative isosorbide is formed by further elimination of water.
The reaction gives about 70 to 80% isosorbide in addition to 30 to 20% undesired by-products, which can be carried out relatively laboriously by distillation, recrystallization from alcohols, recrystallization from the melt, or a combination of these methods or by deposition from the vapor phase. The high purity (> 99.8%) of the isosorbide as a monomer is essential to achieve high molecular weights and uncolored products.
properties
Isosorbide is a white crystalline solid that has a strong water-attracting effect in moist air. The different orientation of the secondary hydroxyl groups in the V-shaped bicyclic system leads to different physical properties and chemical reactivities and therefore allows the selective monoderivatization of isosorbide. The hydroxyl group in the 5-position is endo and forms a hydrogen bond with the oxygen atom in the adjacent furan ring . This makes the hydroxy group in the 5-position more nucleophilic and reactive than the exo -hydroxy group in the 2 position; however, it is more strongly shielded from attack by sterically demanding reactants.
use
Isosorbide
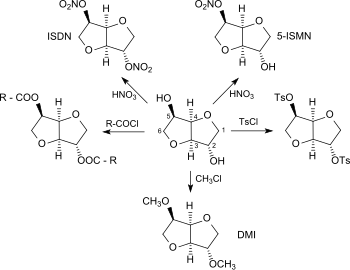
Because of its pronounced hygroscopicity, isosorbide is used as a humectant and in medicine as an osmotic diuretic for the treatment of hydrocephalus and acute narrow-angle glaucoma. The two secondary hydroxyl groups make isosorbide a versatile platform chemical made from renewable raw materials. As a diol, isosorbide can be mono- or bifunctional derivatized with standard reactions of organic chemistry, such as nitration, esterification, etherification, tosylation etc. and converted into compounds with interesting property profiles as well as into monomer building blocks for new types of polymers.
Isosorbide nitrates
The nitration of isosorbide with conc. Nitric acid accessible 2,5- isosorbide dinitrate (ISDN), like its main metabolite 5- isosorbide mononitrate (ISMN ), is suitable for the treatment of angina pectoris due to its vasodilating effect .
Isosorbide ester
When isosorbide is esterified with fatty acids , isosorbide monoesters are available which, because of their surface-active properties, are suitable as detergents in household cleaners, dishwashing detergents and cosmetic preparations. The isosorbide diesters, which are also easily accessible, are used as dispersants for pigments, preservatives, polymer stabilizers, as emulsifiers for cosmetics and as plasticizers for vinyl polymers, in particular polyvinyl chloride (PVC). As a diester of isosorbide and octanoic acid (e.g. obtained from palm oil ), isosorbide dioctanoate consists entirely of bio-based building blocks and has been in use for some time as a particularly non-toxic product as Polysorb (R) ID 37 from Roquette Frères .
Isosorbide ethers
Isosorbide ethers , especially the simplest representative, 2,5-dimethylisosorbide (DMI), are becoming increasingly popular as a sustainable solvent for cosmetic and pharmaceutical preparations and as an electrolyte additive for lithium-ion batteries and as a fuel additive for diesel.
Isosorbide phosphates
Phosphoric acid derivatives of isosorbide are being researched as an environmentally friendly alternative to halogenated flame retardants . So far, 1,2,5,6,9,10- hexabromocyclododecane (HBCD) has been used as a flame retardant in extruded polystyrene rigid foams (XPS) in the construction sector for building insulation, but since May 2013 this has been classified as SVHC ( substance of very high concern ) with a Production and application ban proven. Phosphorus-based isosorbide compounds, such as B. isosorbide bis (diphenyl phosphate), ISTP, into consideration.
ISTP is easily accessible by transesterification of isosorbide with triphenyl phosphate in the presence of potassium carbonate at 150 ° C. The isosorbide bis-diphenyl phosphate obtained as a yellowish oil in 88% yield contains approx. 20% dimers. The high decomposition temperature of ISTP allows it to be used in XPS, although the strong plasticizer effect has a disruptive effect. The flame retardant effect is particularly pronounced in the presence of sulfur-containing synergists, such as. B. bis (diphenylphosphinothionyl) disulfide (BDPS), so that at 3% ISTP the minimum requirement of fire protection class B2 is met.
Polymers made from isosorbide
The now good availability and the high thermal stability of isosorbide make this diol from renewable raw materials interesting as a monomer component for thermoplastic (bio) polymers such as polyesters and polycarbonates , as well as for thermosets such as polyurethanes or epoxy resins . The hydroxyl groups can be converted into primary amino groups via the tosylates and azides, or by addition of acrylonitrile and subsequent hydrogenation with high yield into the corresponding aminopropyl derivatives, which are used as starting materials for diisocyanates - for the production of polyurethanes - as diamines for the production of Polyamides or also as hardeners for epoxy resins are suitable.
The replacement of mono ethylene glycol (MEG) as a diol in the polyester polyethylene terephthalate (PET) leads to polyisosorbide terephthalate (PIT), which is characterized by an extremely high thermal stability (up to 360 ° C under nitrogen). However, the lower reactivity of the secondary hydroxyl groups in the isosorbide results in relatively low molar masses and high residual terephthalic acid contents , which lead to the low chemical stability of the polymers obtained. Therefore, today polyesters with isosorbide and MEG are being investigated as diol components that show improved properties, such as: B. less discoloration.
Isosorbide is of particular interest as a monomer in polycarbonates , where it could replace bisphenol A , which has been identified as a xenoestrogen . The problem with isosorbide-based polycarbonates is their unsatisfactory temperature resistance and impact strength, which is improved by adding comonomers to the isosorbide or by polymer blends.
In polyurethanes , isosorbide itself can serve as a diol or as a building block for the polyol as well as for the diisocyanate component, and also act as a chain extender. By reacting isosorbide with epichlorohydrin , isosorbide-bis-glycidyl ether (a bis-epoxide) is obtained, which can be used as a replacement for the analogous bisphenol A-bis-epoxide with suitable hardeners, such as. B. polyamines or cyclic acid anhydrides can be converted to thermosetting epoxy resins, which are used as adhesives, paints or for coatings on cans for food.
Furthermore, polyoxazolidones are described by the reaction of isosorbide diglycidyl ethers with diisocyanates , which could be used as rigid, highly branched and solvent-resistant thermosets in the electrical and electronics industry.
Isosorbide is a versatile platform chemical made from renewable raw materials, which is now also available in industrial quantities of tens of thousands of tons / year in Europe (Roquette Frères SA) and the USA (Cargill, Inc. and Archer Daniels Midland Co.). The use of isosorbide as a comonomer in PET as a raw material for bottles and as a substitute for bisphenol A , especially in thermoset polycarbonates, is currently considered to be particularly promising .
safety instructions
With an LD 50 value of 24.15 g kg −1 (rat, oral), isosorbide is similarly nontoxic as D - glucose , also with an LD 50 of 25.8 g kg −1 (rat, oral) and is Classified as GRAS (“ generally recognized as safe ”) substance by the Food and Drug Administration FDA .
Individual evidence
- ↑ Entry on ISOSORBIDE in the CosIng database of the EU Commission, accessed on June 30, 2020.
- ↑ a b c d e N.N., Isosorbide as sustainable diol from the C6 platform ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (PDF; 1.6 MB), BioPerspectives 2005, BREW Symposium, Wiesbaden, May 11th 2005
- ↑ Isosorbide data sheet at AlfaAesar, accessed on December 20, 2012 ( PDF )(JavaScript required) .
- ↑ a b Entry on isosorbides in the ChemIDplus database of the United States National Library of Medicine (NLM) , queried on December 18, 2012
- ↑ a b Data sheet Dianhydro-D-glucitol, 98% from Sigma-Aldrich , accessed on January 28, 2013 ( PDF ).
- ↑ a b Isosorbide data sheet at Acros, accessed on January 6, 2013.
- ↑ Patent US9120806 : Dianhydrosugar production process. Published September 1, 2015 , Applicants: Iowa Corn Promotion Board, Inventors: David James Schreck, Marion McKinley Bradford, Nye Atwood Clinton, Paul Aubry.
- ↑ Patent US6670033 : Process and products of purification of anhydrosugar alcohols. Published on December 30, 2003 , Applicant: EI du Pont de Nemours and Company, Inventors: Michael A. Hubbard, Michael Wohlers, Helmut B. Witteler, Edward G. Zey, George Kvakovszky, Thomas H. Shockley, Larry F. Charbonneau, Norbert coal, Jochen Rieth.
- ↑ a b Patent US6867296 : Recovery and purification of anhydro sugar alcohols from a vapor stream. Published on March 15, 2005 , Applicant: EI du Pont de Nemours and Company, Inventor: Kamlesh Kumar Bhatia.
- ↑ G. Flèche, M. Huchette: Isosorbide. Preparation, Properties and Chemistry . In: Starch - strength . tape 38 , no. 1 , 1986, pp. 26–30 , doi : 10.1002 / star.19860380107 .
- ↑ merckmanuals.com: Angle-Closure Glaucoma
- ↑ a b c M. Rose, R. Palkovits, Isosorbide as a Renewable Platform chemical for Versatile Applications - Quo Vadis? , In: ChemSusChem 2012, 5, 167–176 doi: 10.1002 / cssc.201100580 .
- ^ Remington: The Science and Practice of Pharmacy , 21st Edition, p. 1359, edit. DB Troy, Lippincott Williams & Wilkins, 2006, ISBN 0-7817-4673-6 .
- ↑ Pharmacology and Toxicology: For Study and Practice. Published by Claus J. Estler, Harald Schmidt, Schattauer Verlag, 6th edition, 2007, ISBN 978-3-7945-2295-8 .
- ↑ Patent WO2010115565 : Isosorbide monoesters and their use in household applications. Published on October 14, 2010 , Applicant: Cognis IP Management GmbH, inventor C. Breffa et al ..
- ↑ Patent DE102007028702 : Process for the production of dianhydrohexitol diesters. Published on December 24, 2008 , applicant: Evonik Oxeno GmbH, inventor: M. Graß, M. Woelk-Faehrmann.
- ↑ Patent US2012220507 : 2,5-furan dicarboxylate derivatives, and use thereof as plasticizers. Registered on August 17, 2010 , published on August 30, 2012 , applicant: Evonik Oxeno GmbH, inventor: M. Grass, HG Becker.
- ^ Healthy Building Network, Phthalate-free plasticizers in PVC : PDF
- ↑ Grant Industries: About Dimethyl Isosorbide ( Memento of the original from September 7, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent US2010183913 : Lithium cell with iron disulfide cathode and improved electrolyte. Published on July 22, 2010 , Applicant: The Procter & Gamble Co., inventor M. Sliger et al ..
- ↑ Patent US2010064574 : Diesel cycle fuel compositions containing dianhydrohexitols and related products. Published on March 18, 2010 , applicant: Petróleo Brasileiro SA-Petrobras, inventor: RM de Almeida, CR Klotz Rabello.
- ↑ Patent EP2574615 : Process for the production of sugar (thio) phosphates. Published on April 3, 2013 , applicant: BASF SE, inventor: Ch. Fleckenstein, H. Denecke.
- ↑ J. Wagner: Halogen-free flame retardant mixtures for polystyrene foams (PDF; 15.5 MB), Inaugural dissertation, University of Heidelberg, June 2012.
- ↑ Patent US2010130759 : Novel functional compounds with an isosorbide or isosorbide isomer core, production process and uses of these compounds. Published May 27, 2010 , Applicant: Arkema Inc., Inventor: J.-P. Gillet.
- ↑ Patent WO2006032022 : Processes for making low color poly (ethylene-co-isosorbide) terephthalate polymer. Published on August 3, 2006 , applicant: EI du Pont de Nemours and Company, inventor: L. Charbonneau.
- ↑ JC Bersot, et al .: Efficiency Increase of Poly (ethylene terephthalate ‐ co ‐ isosorbide terephthalate) Synthesis using Bimetallic Catalytic Systems . In: Macromol. Chem. Phys. . 212, No. 19, 2011, pp. 2114-2120. doi : 10.1002 / macp.201100146 .
- ↑ MA Hani et al .: Polycondensation of isosorbide and various diols by means of diphosgene characterization by a combination of MALDI and NMR (PDF; 1.7 MB), Recent Res. Devel. Organic Chem., 11 (2007): 1-11, ISBN 978-81-7895-294-9
- ↑ Patent US2011077377 : Method of making isosorbide polycarbonates. Published on March 31, 2011 , Applicant: Sabic Innovative Plastics IP BV, Inventor: H.-P. Brack et al ..
- ↑ Patent US2011160422 : Isosorbide-based polycarbonates, method for making, and articles formed therefrom. Published on June 30, 2011 , Applicant: Sabic Innovative Plastics IP BV, inventor JH Kamps, et al ..
- ↑ PCT patent WO 2012/163845 Fiber composite component and a process for the production thereof , inventor: S. Lindner et al., Applicant: Bayer IP GmbH, published on December 6, 2012
- ↑ X. Feng et al., Sugar-Based Chemicals for Environmentally Sustainable Applications, in Contemporary Science of Polymeric Materials, ACS Symposium Series, Vol. 1061, Chapter 1, pp 3-27, ISBN 978-0-8412-2602-9
- ↑ Frank Bachmann, Joachim Reimer, Marcus Ruppenstein, Joachim Thiem: Synthesis of Novel Polyurethanes and Polyureas by Polyaddition Reactions of Dianhydrohexitol Configurated Diisocyanates . In: Macromolecular Chemistry and Physics . tape 202 , no. 17 , 2001, p. 3410-3419 , doi : 10.1002 / 1521-3935 (20011101) 202: 17 <3410 :: AID-MACP3410> 3.0.CO; 2-Q .
- ↑ Patent US2011015366 : Novel chain extenders for polyurethane elastomer formulations. Published on January 20, 2011 , inventors G. da Costa et al ..
- ↑ Patent US7619056 : thermoset epoxy polymers from renewable resources. Published on November 17, 2009 , Applicant: New Jersey Institute of Technology, inventor AJ East, et al ..
- ↑ Press Release NEWARK, February 24, 2010: NJIT Patent May Be Able To Replace BPA; Make Consumer Products Safer
- ↑ Patent US2010298520 : Polyoxazolidones derived from dianhydrohexitols. Published on November 25, 2010 , Applicant: New Jersey Institute of Technology, inventor AJ East, et al ..
- ↑ AGRICULTURE Project Fact Sheet: New continuous isosorbide production from sorbitol (PDF; 314 kB), Iowa Corn Promotion Board, September 2001
- ↑ D-Glucose data sheet (PDF) from Carl Roth , accessed on August 24, 2010.
- ↑ X. Feng et al., Thermal analysis characterization of isosorbide-containing thermosets, Isosorbide epoxy as BPA replacement for thermosets industry J. Therm. Anal. Calorim., 109 , 1267-1275 (2012), doi: 10.1007 / s10973-012-2581-2