Caprylic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
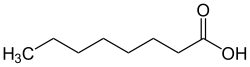
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Caprylic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 16 O 2 | |||||||||||||||||||||
| Brief description |
colorless oil with a slightly rancid odor and a burning taste. |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 144.21 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.9073 g cm −3 (25 ° C) |
|||||||||||||||||||||
| Melting point |
16.5 ° C |
|||||||||||||||||||||
| boiling point |
237 ° C |
|||||||||||||||||||||
| Vapor pressure |
0.25 Pa (298 K) |
|||||||||||||||||||||
| pK s value |
4.89 (25 ° C) |
|||||||||||||||||||||
| solubility |
poor in water (0.68 g l −1 at 20 ° C) |
|||||||||||||||||||||
| Refractive index |
1.4285 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Caprylic acid is the common name for the carboxylic acid octanoic acid, a saturated fatty acid . As with caproic acid and capric acid , the name is derived from the Latin capra or caper for goat or billy goat.
Occurrence and manufacture
Caprylic acid is a triglyceride ( glycerol triester ) in the oil or fat of the coconut ( coconut oil ) to about 5–9%, in butter to about 1.2%. Caprylic acid is also found as a triglyceride in goat butter, milk , palm oil , wine fusel oil , meat products, seafood and cheese . It is found in high concentrations in the seed oils of various species of the cuttlefish ( Cuphea spp.) And the elm family ( Ulmus spp.). The defensive secretion of the flagellum scorpion Mastigoproctus giganteus consists of 5% caprylic acid.
The carboxylic acid is produced synthetically from octanol or octanal by oxidation . A biotechnological production with bacteria ( Escherichia coli ) and yeast ( Saccharomyces cerevisiae ) is possible, though not yet in economically relevant scale.
properties
In its pure form, caprylic acid is a colorless oil with a slightly rancid odor and a burning taste. The carboxylic acid melts at 16.5 ° C and boils at 237 ° C. It is poorly soluble in water , but can be mixed with many organic solvents. Their salts and esters are called caprates and octanoates, respectively. Caprylic acid irritates eyes and skin .
use
Caprylic acid is used in the manufacture of soaps , dyes , natural insecticides, fungicides and antiseptic drugs .
Caprylic acid is also used in insect sprays and advertised as a "natural product" because it is not a classic insecticide . Caprylic acid dissolves the insects' chitin armor , which causes them to die.
Caprylic acid is also used medicinally against fungal infections ( candidiasis ) and some bacterial infections.
Individual evidence
- ↑ Otto Ule , Karl Müller : The nature. Newspaper for the dissemination of scientific knowledge, vol. 8, Halle 1859, p. 171, limited preview in the Google book search.
- ↑ Entry on CAPRYLIC ACID in the CosIng database of the EU Commission, accessed on May 22, 2020.
- ↑ a b c d e entry on octanoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on May 2, 2013.
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-404.
- ↑ a b c d e Entry on octanoic acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ CD Cappa, ER Lovejoy, A .R. Ravishankara: Evaporation Rates and Vapor Pressures of the Even-Numbered C 8 −C 18 Monocarboxylic Acids. In: J. Phys. Chem. A 112, (2008), 3959-3964, doi: 10.1021 / jp710586m .
- ↑ Entry on caprylic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry on Octanoic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Butter in the German Nutrition Advice and Information Network (DEBInet), taken from the Federal Food Code .
- ↑ Octanoic acid at PlantFA Database, accessed November 7, 2017.
- ↑ Jan Gajewski, Renata Pavlovic, Manuel Fischer, Eckhard Boles, Martin Grininger: Engineering fungal de novo fatty acid synthesis for short chain fatty acid production . In: Nature Communications . tape 8 , March 10, 2017, p. 14650 , doi : 10.1038 / ncomms14650 , PMID 28281527 .
- ↑ Zaigao Tan, Jong Moon Yoon, Anupam Chowdhury, Kaitlin Burdick, Laura R. Jarboe: Engineering of E. coli inherent fatty acid biosynthesis capacity to increase octanoic acid production . In: Biotechnology for Biofuels . tape 11 , no. 1 , December 2018, doi : 10.1186 / s13068-018-1078-z , PMID 29619083 .
- ↑ Junjun Wu, Xia Zhang, Xiudong Xia, Mingsheng Dong: A systematic optimization of medium chain fatty acid biosynthesis via the reverse beta-oxidation cycle in Escherichia coli . In: Metabolic Engineering . tape 41 , p. 115–124 , doi : 10.1016 / j.ymben.2017.03.012 ( elsevier.com [accessed May 17, 2019]).

