Nucleation
Nucleation or nucleation is the first sub-process that initiates a first-order phase transition . Examples of this are the freezing of water to ice, the formation of bubbles during the transition from the liquid to the gaseous phase (e.g. when opening a water bottle with dissolved carbon dioxide ) or the condensation of a gas.
General
The essential feature of nucleation is that the new phase , which is thermodynamically stable under the given conditions , is initially formed by nuclei from the old, metastable phase .
The formation of these typically only nanometer- sized nuclei is initially kinetically inhibited . Liquids can be supercooled and overheated . The reason for this inhibition lies in the work that has to be done to form the curved surface of a minute nucleus (e.g. a spherical droplet ) of the new phase. For the smallest droplets or crystals , this surface work is greater than the energy gain from the transition into the new, stable phase. The resulting free energy barrier is called the nucleation barrier and the work that has to be done to overcome this barrier is called the nucleation work (see thermodynamics below). The area of the phase diagram in which the germs remain below the critical germ size is called the Ostwald-Miers area .
However, once nuclei that are larger than the critical nucleus size are formed from thermal fluctuations , they quickly grow to the macroscopic phase. Nucleation can thus also be understood as a prototype of an activated process .
The nucleation rate describes how many nuclei of the new phase are formed per unit of volume and time. This nucleation rate depends exponentially on the work of nucleation: the higher the work of nucleation and thus the barrier to nucleation, the lower the rate (see below kinetics).
Occur
Nucleation is a ubiquitous process. So learns z. For example, the lava ejected by a volcano causes a sudden drop in temperature and pressure and thus forms the typical rocks interspersed with small gas bubbles. Another example are weather phenomena such as the formation of rain , fog and snow . In medicine one knows z. B. diving disease caused by surfacing too quickly; Here the previously dissolved in the blood of nitrogen through the pressure drop outgassed .
Knowledge of the nucleation kinetics is also of great interest to industry. B. to prevent the drop in gas turbines or the boiling delay in evaporators or to control the formation of contrails in jet aircraft . In addition, nucleation plays a central role in the process engineering of polymers , alloys and some ceramics , but also in the control or prevention of crystallization (e.g. in semiconductor materials , metallic glasses or honey ).
Electrofreezing is a process by which the crystallization of water and other liquids during the freezing process can be triggered in a targeted manner by applying an electric field . This physical phenomenon has been known since 1861.
Classical nucleation theory
Because of their great technical relevance, nucleation processes have been systematically investigated since the beginning of the twentieth century. Substantial results have so far been available mainly for the phase transition from gas to liquid, crystallization and for the structural change in a few metals. In the absence of an alternative, the theories put forward for this are often transferred to the remaining systems.
Due to its simple structure, the classical nucleation theory has prevailed to this day , although it has repeatedly been shown, especially for the gas-liquid transition, that its predictions typically deviate from reality by several orders of magnitude (!) B. with argon by more than 20 orders of magnitude. Such large deviations between theory and experiment are almost unique in contemporary science. This is all the more astonishing since it is essentially a problem of classical physics .
Classical nucleation theory makes some basic, simplistic assumptions that allow a description of the process. A large part of these approximations can be summarized under the term 'capillarity approximation': With this approximation it is assumed that even the smallest ( microscopic ) nuclei already have the same (macroscopic) properties of the new phase.
To illustrate the classical nucleation theory, the example of the condensation of a droplet from a supersaturated gas phase is used below . In this case, the process is usually viewed at constant temperature and the driving force is supersaturation.
thermodynamics
In the case of a supersaturated gas phase, the current pressure is higher than the equilibrium vapor pressure at a given temperature :
The reversible work that is necessary to form a liquid droplet from this supersaturated gas phase, i.e. H. the work of nucleation can be calculated. For this purpose, a process with constant pressure , constant volume and constant number of particles is considered. The relevant thermodynamic potential is therefore the Gibbs free energy :
with the chemical potential .
Specifically for a pure vapor phase (index v for vapor ) results:
- .
For a system that contains both vapor and a liquid droplet (index l for liquid ) made of particles, we get:
- ,
where is the surface tension of a droplet with the surface .
The work of nucleation for a droplet of the size in the gas phase is the difference between the Gibbs free energy of a system containing a droplet of the size and steam and that of the pure steam system :
- .
In this equation is the difference between the chemical potentials of the vapor and the liquid.
The classical nucleation theory makes a number of simplifying assumptions:
- The liquid droplet is spherical, incompressible and has a sharp interface .
- The liquid droplet has the same surface tension, density and vapor pressure as the macroscopic (flat) liquid phase.
- The vapor pressure can be described with the help of the ideal gas law .
With the help of these assumptions and the Gibbs-Duhem equation , the difference in chemical potentials can be calculated:
- .
With
- the Boltzmann constant
- of oversaturation .
The surface can also be calculated:
With
- the radius of the droplet
- the mean surface area of a particle (molecule)
- the mean volume of a particle in the liquid phase.
This results in the work of nucleation
- .
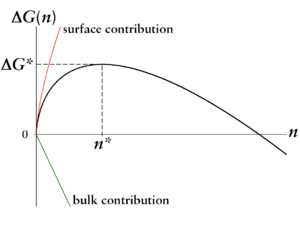
The first term, also called the volume term, is proportional to . It represents the gain (therefore negative sign) in energy that occurs when a molecule passes from the metastable vapor phase to the stable liquid phase.
The second term is the work that needs to be done (hence positive sign) to shape the surface of such a liquid droplet. He is proportional to .
With - only in this case can nucleation occur - the surface term for small numbers of particles or droplet sizes dominates. From a critical droplet size , the volume term gains the upper hand. The maximum is the work of nucleation that must be applied to form a droplet of the critical size. This gives the picture shown of a barrier in the free energy .
The critical size and the critical nucleation work are the determining parameters of the nucleation:
- Droplets smaller than have a higher probability of evaporating again than growing further, since for them the evaporation is associated with a gain in Gibbs free energy (movement down the curve).
- Only droplets larger than have a higher probability of growing further than evaporating and can thus serve as seeds for the new phase.
So it is understandable why a substance can be kept metastable for a longer period of time: Although the new phase (in our example the liquid) is the thermodynamically stable phase, the process first has to go through some energetically unfavorable steps in order to climb the barrier.
The barrier and the critical nucleus size according to the classical nucleation theory are:
and
- .
kinetics
In order to calculate a nucleation rate, i.e. the number of nuclei formed per volume and time, the kinetics of nucleation must also be considered. The stationary nucleation rate is usually calculated using an Arrhenius approach :
The pre-factor is usually called the kinetic pre-factor, but it is not discussed in detail here. The height of the barrier usually has a much greater and decisive influence due to the exponential dependence.
Types of nucleation
Homogeneous nucleation
If the nucleation takes place in free space, i.e. through a static meeting of the same particles, one speaks of homogeneous nucleation.
For this it is necessary that, in the case of condensation, a sufficient number of slow particles come together to form larger structures without further help. Slow particles can arise from the simultaneous collision of more than two particles ( triple collision ). One particle absorbs a large part of the kinetic energy and leaves two slow particles behind. The supersaturation is roughly proportional to the probability of such a three-way collision that leads to nucleation. Depending on the system under consideration, thermodynamically metastable systems can therefore remain in this state for a very long time.
Heterogeneous nucleation
In contrast to homogeneous nucleation, only very low supersaturations of often less than one percent are required with heterogeneous nucleation. In the case of condensation, this form of condensation takes place on existing surfaces (heterogeneous: on particles of a different type ), i.e. usually on solid particles that float in the gas phase, the condensation nuclei or aerosol particles . These act as a kind of particle catcher in relation to the respective gas, with the radius and chemical properties of the particle essentially determining how well the gas particles adhere to it. This also applies analogously to surfaces of non-particulate bodies, which is then referred to as fitting . In any event, heterogeneous particles or surfaces act as a nucleation catalyst by significantly lowering the nucleation barrier.
See also
Individual evidence
- ↑ M. Volmer and A. Weber: Droplet formation in steams, Z. Phys. Chem. (Leipzig) Vol. 119, p. 227, 1926
- ↑ L. Farkas: Nucleation rate in supersaturated vapors, Zeitschr. f. physics. Chemie, Vol. 125, p. 236, 1927 PDF
- ↑ R. Becker, W. Döring: Kinetic treatment of nucleation in supersaturated vapors, Ann. Phys. Vol. 24, p. 719, 1935, doi : 10.1002 / andp.19354160806
- ↑ D. Kashchiev, Butterworth-Heinemann (ed.): "Nucleation: Basic Theory With Applications", 2000, ISBN 0-7506-4682-9
- ↑ PG Debenedetti, Princeton University Press (Ed.): "Metastable Liquids: Concepts and Principles", 1996
- ↑ Alexander Fladerer, Reinhard Strey: Homogeneous nucleation and droplet growth in supersaturated argon vapor: The cryogenic nucleation pulse chamber. In: The Journal of Chemical Physics. 124, 2006, p. 164710, doi : 10.1063 / 1.2186327 .
- ↑ A. Fladerer: "Nucleation and droplet growth in supersaturated argon vapor: Construction of a cryogenic nucleation pulse chamber", Cuvillier-Verlag, Göttingen (2002), ISBN 3-89873-494-3 . PDF
Web links
- The Extreme Diet Coke & Mentos Experiments (en) - Applied Nucleation