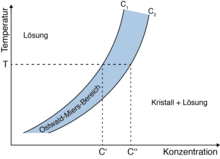
Ostwald-Miers area
The Ostwald-Miers range (according to Wilhelm Ostwald and Henry Alexander Miers ) is the temperature range in which the melting point falls below the melting point during cooling in a liquid or the solubility product is exceeded in a solution without spontaneous crystallization . In other words, it is the temperature range in which the melt can be kept in the supercooled state or the solution in the supersaturated state.
It is the temperature range between the melting point of the crystal and the melting point of the crystal nuclei .
A liquid or solution in the Ostwald-Miers area is thermodynamically metastable and only crystallizes when seed crystals are added or further subcooling.
Similarly, there is an Ostwald-Miers range not only in the transition from the liquid to the solid phase, but also in the transition from the gas phase to the liquid or solid - regardless of which of the state variables in the phase diagram the transition takes place.
The area exists because a nucleus of sufficient size must spontaneously arise for crystallization. Microscopic nuclei or ordered clusters of molecules are permanently formed spontaneously in a solution or melt. As long as the surface energy ( ~ r 2 ) and, to a small extent, the elastic energy (e.g. tension energy) are greater than the energy released during crystallization (~ r 3 ), these spontaneously formed molecular arrangements disintegrate. Only above a critical nucleus radius does the nucleus continue to grow and crystallization occurs. According to this view, the area can also be narrowed down to the area between the upper phase boundary (e.g. melting point) and the area in which nuclei with sufficiently large nucleus radii always arise. For a more quantitative description of the liquid phase / gas phase case, see section critical radius under Kelving equation.
A widespread application in which a broad Ostwald Miers area is exploited are hand warmers or heating pads with sodium acetate - trihydrate filling. Here, a fault (buckling of a metal plate) forms a nucleus with a sufficient size above the critical nucleus radius and crystallization occurs.
When Czochralski method for the preparation of silicon - single crystal , the temperature of the liquid silicon within the Ostwald-Miers region is maintained, in order to ensure an undisturbed and offset-free crystallization.
Individual evidence
- ^ LJ Spencer : Biographical notice of Sir Henry A. Miers (1858-1942) . In: Journal of the Mineralogical Society . No. 185 , 1944, pp. 17-28 ( PDF ).
- ↑ GMV: Ostwald-Miers area. In: Lexicon of Geosciences. Spektrum der Wissenschaft Verlagsgesellschaft mbH / Spektrum Akademischer Verlag , 2000, accessed on July 12, 2016 .