High performance plastics

High-performance plastics are a subgroup of thermoplastics that differ from engineering plastics and standard plastics in particular in their temperature resistance , but also in terms of chemical resistance and mechanical properties . At the same time, however, they are also more expensive and are produced in smaller quantities.
Definition of terms
There are numerous synonyms for the term high-performance plastics, such as: high-temperature plastics, high-performance polymers, high-performance thermoplastics or high-tech plastics. The name high-temperature plastics comes from the fact that their long-term use temperature is by definition higher than 150 ° C (even if this is not their only characteristic, see above). Polymers are often used instead of plastics , as both terms are often used synonymously by users. The term high-performance thermoplastics emphasizes that thermosets and elastomers are outside the classification of plastics into standard, technical and high-performance plastics and form their own classes .
A comparison of standard plastics, engineering plastics and high-performance plastics is illustrated by the following figure (the abbreviations can be clicked):
The differentiation from less high-performance plastics varies over time: While nylon and poly (ethene terephthalate) were considered exceptionally high-performance plastics when they were first introduced, they are now considered to be extraordinarily common.
history
Improving mechanical properties and temperature resistance has always been an important goal in research into new plastics. Since the early 1960s was the development of high-performance plastics through appropriate needs in the air - and space as well as in nuclear technology advanced. For example, synthetic routes for PPS , PES and PSU were developed by Philips , ICI and Union Carbide in the 1960s . The market entry always took place in the early 1970s. A manufacture of PEEK (ICI), PEK (ICI) and PEI (General Electrics and GE) via polycondensation was developed in the 1970s. PEK was already available from Raychem in 1972 , but it was produced via an electrophilic synthesis. Since electrophilic syntheses generally have the disadvantage of a low selectivity towards linear polymers and use aggressive starting materials, the product was only able to stay on the market for a short time. For this reason, the majority of high-performance plastics today are manufactured using polycondensation processes. In manufacturing processes through polycondensation, a high degree of purity of the starting materials is important. In addition, stereochemistry plays a role in achieving the desired properties. The development of new high-performance plastics is therefore closely linked to the development and economic production of the underlying monomers .
properties
High-performance plastics meet higher demands than standard and engineering plastics, as they have better mechanical properties, higher chemical and / or higher heat resistance. The latter, however, also complicates their processing , and special machines are often necessary. In most cases, high-performance plastics specialize in a single property (e.g. heat resistance ). This is in contrast to engineering plastics, which cover a wide range of functions.
Both the mechanical and thermal properties of high-performance plastics are due to their molecular structure (structure-property relationship).
Structure-property relationship
All high-performance plastics contain aromatic structures . Aromatic structures combine the two most important features for resistance to high temperatures: On the one hand, they are resistant to oxidation, since the aromatic CH bond with 435 kJ · mol −1 is significantly more stable than aliphatic CH bonds with 350–400 kJ · mol −1 . This impedes the formation of radicals that occur in the event of thermal decomposition or fire . On the other hand, the chain stiffness of aromatic polymers is greater than that of aliphatic polymers, which increases the glass transition temperature T g (and the crystalline melting point T m in the case of crystalline polymers) and decreases the solubility .
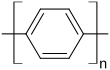
A polymer made from purely aromatic units such as poly ( p -phenylene)
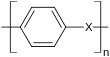
has an extremely high softening point of around 500 ° C (compared to polyethylene of 110 ° C) and can still be used at high temperatures. However, this makes the processability very difficult. In order to find a compromise between processability and stability, functional groups X (also temperature-resistant) are present in all commercial high-performance plastics :
X can be various flexible groups such as an ether group as a diphenyl ether group e.g. B. in polyether ketones or a sulfur atom as a diphenyl sulfide group z. B. in polyphenylene sulfide (PPS). It is also possible to incorporate more rigid groups, such as a sulfur atom as a diphenylsulfone group in PES or a nitrogen atom as an imide group in polyetherimide (PEI) or polyamideimide (PAI).
Mechanical properties
| Density [g · cm −3 ] | Melting temperature [° C] | HDT / A [° C] | Modulus of elasticity [ MPa] | Yield stress [ MPa] | CTI / A [-] | |
|---|---|---|---|---|---|---|
| PE-LD | 0.915-0.935 | 105-118 | - | 200-400 | 8-10 | 600 |
| SECTION | 1.03-1.07 | 235 | 95-105 | 2200-3000 | 45-65 | 550-600 |
| PA 6.6 | 1.13-1.15 | 255-260 | 70-100 | 2700-3300 | 75-100 | 600 |
| PEEK | 1.30 | 343 | 152 | 3500 | 100 | 150 |
| PES | 1.36-1.37 | - | 200-205 | 2600-2800 | 75-80 | 100-150 |
| PAI | 1.38-1.40 | - | - | 4500-4700 | 275 | 175 |
The table shows some properties of the standard plastics low-density polyethene (PE-LD) and acrylonitrile-butadiene-styrene (ABS), the engineering plastic polyamide 6.6 (PA 6.6) and the high-performance plastics polyetheretherketone (PEEK), polyethersulfone (PES) and polyamideimide ( PAI) (both unamplified ). It can be seen that the density, the thermal properties (melting temperature and HDT / A), the mechanical properties (modulus of elasticity and yield stress) and the electrical properties (CTI) increase towards the high-performance plastics. Some values are so high or so low that they can no longer be measured and are therefore missing from the table.
Thermal stability
Thermal stability is a central property of high-performance plastics. Based on the properties of standard plastics, certain mechanical and thermal improvements can be achieved by adding reinforcing materials (e.g. glass and carbon fibers ), adding stabilizers and increasing the degree of polymerisation . Usage temperatures of up to 130 ° C are achieved by replacing aliphatic with aromatic units. A similar temperature resistance is achieved with up to 150 ° C of thermosetting plastics (which do not belong to the high-performance plastics, see above). An even higher service temperature can be achieved by completely dispensing with aliphatic elements and tightly linking aromatics with functional groups such as ether , sulphone or imide groups . This allows usage temperatures of 200 ° C in the case of polyethersulfone (PES) to 260 ° C in the case of polyetherimide (PEI) or polyamideimide (PAI).
The increase in temperature stability through the incorporation of aromatics is related to the fact that the temperature stability of a polymer is determined by its resistance to thermal degradation and its resistance to oxidation . The thermal degradation occurs primarily through a statistical chain splitting; Depolymerization and elimination of low molecular weight compounds only play a subordinate role. The thermal-oxidative degradation of a polymer begins at lower temperatures than the purely thermal degradation. Both types of degradation take place via a radical mechanism. Aromatics offer good protection against both types of degradation, since free radicals are delocalized by the Π-electron system of the aromatics and thus stabilized and since the aromatic binding energy is particularly high (see above). The thermal stability increases so strongly.
In practice, the highest temperature resistance (about 260 ° C) is achieved with fluoropolymers in which the hydrogen atoms of the hydrocarbons have been replaced by fluorine atoms . PTFE has the largest market share here with 65–70%. However, fluorine-containing polymers are not suitable as construction materials because of their poor mechanical properties (low strength and rigidity, strong creep under load). They are therefore not always counted among the high-performance plastics.
Crystallinity
Like all polymers, high-performance plastics can be divided into amorphous and semi-crystalline ( see figure above ). This helps to break down some general characteristics.
Crystalline polymers (especially those that are reinforced with fillers ) can also be used above their glass transition temperature . This is due to the fact that, in addition to the glass transition temperature T g , partially crystalline polymers also have a crystalline melting temperature T m , which is usually significantly higher. For example, PEEK has a T g of 143 ° C, but can still be used up to 250 ° C (continuous service temperature (CST) = 250 ° C). Another advantage of semi-crystalline polymers is their high resistance to chemically aggressive substances: PEEK, for example, is highly resistant to aqueous acids , alkalis and organic solvents .
Solution properties
High-performance plastics are generally only sparingly soluble. Some, such as poly (ether ether ketone) (PEEK), are only soluble in strong acids (such as concentrated sulfuric acid ), which leads to sulfonation .
use
High-performance plastics are relatively expensive: The price per kilogram can be between 5 ( PA 4.6 ) and 100 US dollars ( PEEK ). The average value is a little less than $ 15 / kg . High-performance plastics are therefore around 3 to 20 times more expensive than engineering plastics. No significant price decline is to be expected here in the future either, as it can be assumed that the investment costs for production systems, the complex development and the high sales costs will remain constant. At 749,000 t / year (as of 2014), their production volume is very low, their market share is around 1%.
Among the high-performance polymers, fluoropolymers have a 45% market share (main representatives: PTFE ), sulfur-containing aromatic polymers 20% market share (PPS), aromatic polyaryl ethers and polyketones 10% market share (PEEK) liquid crystal polymers (LCP) with 6%. 41% of high-performance plastics are used in the electrical and electronics industry and 24% in the automotive industry, making them the largest consumers. All other industries (including the chemical) have a share of 23%.
Examples
The high-performance plastics include:
- Polyaryls in which aromatic rings are linked via oxygen or sulfur atoms or CO or SO 2 groups. These include polyphenylene sulfides , polyether sulfones and polyether ketones
- Aromatic polyester (polyarylate) and polyamides (polyaramides) such as poly- m -phenylenisophthalamid (PMI)
-
Heterocyclic polymers such as polyimides, polybenzimidazoles and polyetherimide
- Polyimides are heterocyclic polymers that are stable up to 550 ° C. Polyimides are very resistant to chemicals and are suitable as work pieces in mechanical engineering (valves, bearings) and in electrical engineering for bobbins and as cable insulation and substrates for printed circuit boards.
- Another class, the polyetherimides , has a lower softening point (approx. 360 ° C) and can be processed using standard processes in the plastics industry (injection molding).
- Polybenzimidazoles (PBI) can be used for protective suits and fireproof fabrics in aircraft.
- Aromatic polyamides based on poly- m -phenylenisophthalamid (PMI) are suitable for spinning from solution. This fiber has a very high tear strength and good temperature stability. Only at around 400 ° C does the fiber deform and char. Polyaramid fibers are used as protective clothing against heat or as interior lining in aircraft.
- Liquid crystal polymers (LCP Liquid Crystalline Polymers).
- Fluoropolymers such as polytetrafluoroethylene (PTFE, called on Teflon) or perfluoroalkoxylalkane (PFA) with a temperature resistance of up to 260 ° C. However, fluoropolymers are only classified as high-performance plastics in some cases.
- Polyphenylenes.
literature
- Patrick E. Cassidy, Tejraj M. Aminabhavi, V. Sreenivasulu Reddy: Heat-Resistant Polymers (= Kirk-Othmer Encyclopedia of Chemical Technology). John Wiley & Sons, Inc., December 4, 2000, ISBN 0471238961 , doi : 10.1002 / 0471238961.0805012003011919.a01 .
- MM Sayyed: Studies in synthesis and characterization of polycondensation polymers ( English ) In: Dissertation, archived at Shodhganga . Shivaji University. Retrieved December 13, 2015.
Individual evidence
- ↑ a b c Hans-Georg Elias: Makromoleküle, Volume 4: Applications of polymers . 6th edition. Wiley-VCH, Weinheim 2003, ISBN 3-527-29962-9 , pp. 298 ( limited preview in Google Book search).
- ↑ a b c d e f g David Parker, Jan Bussink, Hendrik T. van de Grampel, Gary W. Wheatley, Ernst-Ulrich Dorf, Edgar Ostlinning, Klaus Reinking, Frank Schubert, Oliver Jünger: Polymers, High-Temperature . In: Ullmann's Encyclopedia of Industrial Chemistry . April 2012. doi : 10.1002 / 14356007.a21_449.pub3 .
- ^ A b Wolfgang Kaiser : Plastics chemistry for engineers: From synthesis to application . 2nd Edition. Carl Hanser, 2007, ISBN 978-3-446-41325-2 , pp. 439 ( limited preview in Google Book search).
- ^ A b R. W. Dyson: Specialty Polymers . Chapman and Hall, New York 1987, ISBN 0-216-92248-8 .
- ↑ Material data sheet ABS. Retrieved July 18, 2015 .
- ↑ Product Data Sheet Duratron PAI. Retrieved July 18, 2015 .
- ↑ a b c Walter Hell Erich Günther Harsch, Erwin Baur: Material Guide Plastics: properties, tests, parameters . 10th edition. Carl Hanser Verlag, Munich 2010, ISBN 978-3-446-42436-4 , pp. 1 ( limited preview in Google Book search).
- ↑ Gottfried W. Ehrenstein, Sonja Pongratz: Resistance of plastics . Carl Hanser Verlag, Munich 2007, ISBN 978-3-446-21851-2 , p. 38–47 ( limited preview in Google Book search).
- ^ A b Wilhelm Keim: Plastics: Synthesis, Manufacturing Processes, Apparatus . 1st edition. Wiley-VCH, Weinheim 2006, ISBN 3-527-31582-9 , pp. 214 ( limited preview in Google Book search).
- ^ Matthew T. Bishop, Frank E. Karasz, Paul S. Russo, Kenneth H. Langley: Solubility and properties of a poly (aryl ether ketone) in strong acids . In: Macromolecules . tape 18 , no. 1 , January 1, 1985, ISSN 0024-9297 , pp. 86-93 , doi : 10.1021 / ma00143a014 .
- ↑ a b c d KIweb.de plastic information. Retrieved January 24, 2014 .
- ↑ "Almost one million tons of high-performance plastics" in k-zeitung.de. (No longer available online.) Archived from the original on July 10, 2015 ; Retrieved July 18, 2015 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b c Harald Cheldron, Friedrich Herold, Arnold Scheller: Technically important temperature- resistant polymers . In: Chemistry in Our Time . 23, No. 6, December 1989, pp. 181-192. doi : 10.1002 / ciuz.19890230602 .