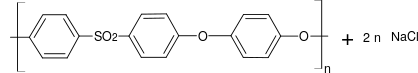
Polysulfones

Polysulfones are a class of high-performance thermoplastic plastics . They are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl -SO 2 -aryl subunit. Due to the high material and processing costs, polysulfones are only used in special applications, often as a superior alternative to polycarbonates .
Three polysulfones are used industrially, these are polysulfone (PSU), polyethersulfone (PES) and polyphenylene sulfone (PPSU). They can be used in the temperature range from −100 ° C to +200 ° C and are used for electrical devices, in vehicle construction and in medical technology . They are made up of para-linked aromatics , sulfone and ether groups and, in some cases, also alkyl groups .
Polysulfones have excellent heat and oxidation resistance , hydrolysis- resistant against aqueous and alkaline media and good electrical properties.
Definition and technically used polysulfones
In principle, any polymer that contains a sulfone group could be referred to as a "polysulfone". When “polysulfones” are mentioned, however, mostly polyaryl ether sulfones (PAES) are meant, since only aromatic polysulfones are used as technical material. Since there are always ether groups in the technically used polysulfones, PAES are also referred to as poly (arylene sulfone) s, as polyether sulfone (PES) or simply polysulfone (PSU). The three terms (and abbreviations) can therefore be synonyms . As a name for all polysulfones, “Poly (arylethersulfone) e (PAES)” is to be preferred, since polysulfone (PSU), polyethersulfone (PES) and poly (arylene sulfone) (PAS) are also used as names for individual polymers. These and some other PAES are listed in the table in the chapter Technically relevant polysulfones .
history
The simplest polysulfone, poly (phenylene sulfone), was already known before 1960. It can be represented in a Friedel-Crafts reaction from phenyl sulfone chloride:
Since this polymer has a melting point of over 500 ° C, it is very heat-resistant on one side, but at the same time very difficult to process on the other. In addition, its mechanical properties are rather poor. For this reason, research was carried out on thermoplastically (from the melt ) processable polysulfones at that time . It has already been assumed that polyaryl ether sulphes (PAES) would be suitable for this purpose.
Corresponding synthetic routes to PAES were developed almost simultaneously and nevertheless independently of one another by 3M Corporation, Union Carbide Corporation in the USA and in the Plastics Division of ICI in Great Britain. The polymers found at the time are still used today, but produced using a different synthesis process.
The synthesis process used at that time was via an electrophilic synthesis. Not only para but also ortho bonds were created, which in some places led to crosslinking and generally to poorer mechanical properties.
The syntheses consisted of an electrophilic aromatic substitution of an aryl ether with a sulfuryl chloride using a Friedel-Crafts catalyst (e.g. iron (III) chloride , antimony (V) chloride ):
All PAES commercially available today are not represented via this synthesis, but rather a nucleophilic synthesis, see chapter Production .
Manufacturing
Technically, polyether sulfones are produced through a polycondensation reaction of an aromatic dihydroxy component (e.g. the sodium salt of bisphenol A ) and a bis (halophenyl) sulfone. The sodium salt of the dihydroxy component is formed in situ by a reaction with the stoichiometric amount of sodium hydroxide (NaOH).
The resulting water must be removed with an azeotropic solvent (e.g. methylbenzene or chlorobenzene ), otherwise problems will arise. The polymerization takes place at 130-160 ° C under inert conditions in a polar, aprotic solvent, e.g. B. Dimethyl sulfoxide :
As bis (halophenyl) sulfone, difluorides can be used; they are more reactive than dichlorides, but too expensive for commercial use. Chain terminators ( chloromethane ) can be used to regulate the chain length within a range that enables technical melt processing . However, the product according to the reaction equation below still has reactive end groups. In order to prevent further condensation in the melt, the end groups are z. B. etherified with chloromethane.
properties
Polysulfones are amorphous plastics that are rigid, high-strength and very transparent. They are also characterized by high strength, rigidity and hardness and retain these properties even between −100 and 150 ° C. The glass transition temperature of polysulfones is 190 to 230 ° C. The dimensional stability is very high, the change in size on contact with boiling water, with hot air or steam at 150 ° C is less than 0.1%.
Polysulfones are also characterized by good chemical resistance. They are very resistant to mineral acids and alkalis (in a pH range from 2 to 13) as well as to electrolytes . They are not resistant to non-polar organic solvents (e.g. ketones and chlorinated hydrocarbons ) and aromatic hydrocarbons, they dissolve in dichloromethane and methylpyrrolidone .
Polysulfones are counted among the high-performance plastics. They can be processed by injection molding, extrusion or hot forming.
Structure-property relationship
Poly (aryl ether sulfone) s are built up from aromatics, ether groups and sulfonic acid groups. Poly (phenylene sulphone), which consists only of sulfonic acid groups and phenyl groups, can serve as a comparison for the function of the individual components. Since both groups are thermally very stable, poly (phenylene sulphone) has an extremely high melting temperature (520 ° C). At the same time, however, the polymer chains are so rigid that poly (phenylene sulphone) (PAS) decomposes before melting and therefore cannot be processed thermoplastically. Therefore flexible elements have to be built into the chains, this is done in the form of ether groups . They allow the polymer chains to rotate freely. This leads to a significantly reduced melting point and also improves the mechanical properties through increased impact strength. The alkyl groups in bisphenol A also act as a flexible element. The reason for the stability of the polymer can also be traced back to the individual structural elements:
The sulfone group, in which sulfur is in the highest possible oxidation state , attracts electrons from the neighboring benzene rings and causes a lack of electrons there . The polymer therefore resists further electron loss, which explains the high resistance to oxidation. The sulfone group is also linked to the aromatic by mesomerism via a strong bond (bond reinforced by mesomeric energy). As a result, larger amounts of energy from heat or radiation can be absorbed by the molecular structure without any reactions ( decomposition ) occurring. The mesomerism also has the consequence that the configuration is particularly rigid. Thanks to the diphenylsulfone group, the polymer is permanently heat-resistant, resistant to oxidation and still has a high degree of rigidity even at elevated temperatures. The ether bond provides (in contrast to esters ) resistance to hydrolysis and a certain flexibility which leads to impact strength. In addition, the ether bond leads to good heat resistance and better flowability in the melt.
use
Polysulfone has one of the highest operating temperatures of all thermoplastics that can be processed from the melt. Due to its resistance to high temperatures, it can be used as a flame retardant without its mechanical properties being impaired (as is the case with many other flame retardants). Its high hydrolytic stability allows it to be used in medical applications that require sterilization in an autoclave . However, it is prone to some solvents and weathering ; however, the instability to weathering can be compensated for by adding other materials.
Polysulfones enable the simple production of membranes with reproducible properties and controllable pore size , up to 40 nanometers. Such membranes can be used in applications such as hemodialysis , wastewater recovery , food and beverage processing, and gas separation. They are also used in the automotive and electronics industries. Filter cartridges made from polysulfone membranes offer extremely high flow rates even at very low pressure differences compared to nylon or polypropylene .
Polysulfones can be reinforced with glass fibers . The resulting composite material has twice the tensile strength and three times the modulus of elasticity .
Technically relevant polysulfones
Some technically relevant polysulfones are listed in the following table:
| Structural formula | Name (brand name, company) | Chemical name | CAS |
|---|---|---|---|
| Polyarylene sulfone (PAS) | |||
| Polybisphenyl sulfone (PSF) | Poly [oxy-1,4-phenylenesulfonyl-1,4-phenyleneoxy-1,4-phenylene (1-methylethylidene) -1,4-phenylene] | 25135-51-7 | |
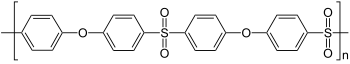
| Polyethersulfone (PES) | Poly (oxy-1,4-phenylsulfonyl-1,4-phenyl) | 25608-63-3 | |
| Polyphenylene sulfone (PPSU) | 25608-64-4 | ||
| Polysulfone (PSU) | Poly (oxy-1,4-phenylenesulfonyl-1,4-phenylene) | 25667-42-9 | |
| Victrex HTA | 121763-41-5 |
Individual evidence
- ↑ Wolfgang Kaiser : Kunststoffchemie für Ingenieure: From synthesis to application . 2nd Edition. Carl Hanser, 2007, ISBN 978-3-446-41325-2 , pp. 461 ( limited preview in Google Book search).
- ^ A b R. Becker, Ludwig Bottenbruch, Rudolf Binsack, Gerhard W. Becker, Dietrich Braun: high-performance plastics. Polyarylates, thermotropes, polyesters, polyimides, polyetherimides, polyamideimides, polyarylene sulfides, polysulfones, polyetheretherketones. Plastic manual volume 3/3. Technical thermoplastics . Ed .: Gerhard W. Becker, Dietrich Braun, Ludwig Bottenbruch. tape 3/3 . Hanser, Munich [a. a.] 1994, ISBN 3-446-16370-0 , pp. 140 ( limited preview in Google Book search).
- ^ Johannes Karl Fink: High Performance Polymers (= Industrial polymer technology and applications ). Andrew, Norwich, NY 2008, ISBN 978-0-8155-1580-7 , pp. 453–481 (English, limited preview in Google Book Search).
- ↑ Lechner, Manfred D .; Gehrke, Klaus; Nordmeier, Eckhard: Macromolecular chemistry: a textbook for chemists, physicists, materials scientists and process engineers . 4th edition. Birkhäuser, Basel; Boston, Mass .; Berlin 2010, ISBN 978-3-7643-8890-4 , pp. 134 ( limited preview in Google Book search).
- ↑ a b Patent GB1060546 : Polyarylsulphone polymers. Applicant: MINNESOTA MINING & MFG, inventor: HA Vogel.
- ↑ Patent GB1078234 : Polyarylene Polyethers. Filed 1973 , applicant: Union Carbide Corporation, inventor: Alford G. Farnham, Robert N. Johnson.
- ↑ patent GB1153035 : Production of Aromatic Polymers and Intermediates therefor. Applicant: ICI LTD, inventor: BARR DENNIS ARTHUR; ROSE JOHN BREWSTER.
- ↑ a b J.B. Rose: Preparation and properties of poly (arylene ether sulphones) . In: polymer . 15, No. 7, July 1974, pp. 456-465. doi : 10.1016 / 0032-3861 (74) 90111-6 .
- ↑ Handbook of Biomaterial Properties . Springer Science & Business Media, 1998, ISBN 978-0-412-60330-3 , pp. 283 ( books.google.de ).
- ↑ Hee-Gweon Woo, Hong Li: Advanced Functional Materials . Springer Science & Business Media, 2011, ISBN 978-3-642-19077-3 , p. 23 ( books.google.de ).
- ↑ David Parker, Jan Bussink, Hendrik T. van de Grampel, Gary W. Wheatley, Ernst-Ulrich Dorf, Edgar Ostlinning, Klaus Reinking, "Polymers, High-Temperature" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH: Weinheim . doi : 10.1002 / 14356007.a21_449
![{\ mathrm {Ar-SO_ {2} -Cl \ longrightarrow - [- Ar-SO_ {2} -] _ {n} + HCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cff5694b952876992b1d382e5d280809bdc5e113)