Electron configuration
The electron configuration indicates the distribution of electrons in the electron shell of an atom to different energy states or lounges ( atomic orbitals ).
Quantum numbers and shells
The state of each electron in the shell is determined according to the Bohr-Sommerfeld atomic model and the orbital model using four quantum numbers :

| Quantum number | character | Range of values | designation | Examples |
|---|---|---|---|---|
| Principal quantum number | 1, 2, 3, ... | K, L, M, ... | 3 | |
| Minor quantum number | 0,…, n − 1 | s, p, d, f, ... | 0, 1, 2 | |
|
magnetic angular momentum quantum number |
−l, ..., + l | s, p x, y, z , d yz, xz, xy, z², x²-y² , ... |
−2, −1, ± 0, +1, +2 | |
|
magnetic spin quantum number |
−½, + ½ | ↓, ↑ | −½, + ½ |
According to the Pauli principle , no two electrons of an atom must have the same state in all four quantum numbers. With this principle it can be shown that the electrons are distributed to the various permitted states and thus to the shells and subshells.
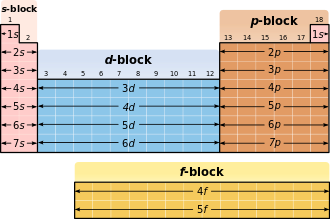
The main quantum numbers determine the shells, the secondary quantum numbers the sub-shells. Each shell, according to the limitations of , and be filled with a maximum 2n² electrons. The shells are designated in ascending order, starting with the core shell, with capital letters: K, L, M, N, O, P, Q ... The orbitals are named according to the series of spectral lines that an excited electron emits when it is in original orbital falls back; For historical reasons, the first four series are called s (“sharp”), p (“principal”), d (“diffuse”) and f (“fundamental”).
The outermost occupied shell ( valence shell ) determines the chemical behavior and is therefore decisive for the classification in the periodic table .
Filling the bowls according to the build-up principle

As the number of electrons in the elements increases , the possible states - starting with the lower energies - are occupied. According to Hund's rule , the orbitals of the same energy are first assigned once, then twice.
The sub-shells are filled in the following order (in rows, i.e. in periods):
- 1st period : 1s
- 2nd period : 2s 2p
- 3rd period : 3s 3p
- 4th period : 4s 3d 4p
- 5th period : 5s 4d 5p
- 6th period : 6s 4f 5d 6p
- 7th period : 7s 5f 6d ...
Relation to the periodic table
In the periodic table, the occupation of the s orbital of a new shell corresponds to the jump into a new period .
| Orbital / block |
Number of electrons |
includes elements of ... |
|---|---|---|
| s | 2 | Elements of the 1st and 2nd main group as well as helium |
| p | 6th | remaining main group elements |
| d | 10 | all subgroup elements |
| f | 14th | all lanthanoids and actinides |
notation
| 2p |
|
||||
|---|---|---|---|---|---|
| L. | 2s |
|
|||
| K | 1s |
|
|||
| 1s 2 2s 2 2p 4 [He] 2s 2 2p 4 |
|||||
The electron configuration of an atom is described by the occupied subshells:
- The number of the shell is followed by the letter for the lower shell and superscript the number of electrons in the lower shell. So z. B. for the 2nd lower shell (l = 1 or p) occupied by 5 electrons of the 3rd shell (n = 3 or M) the notation 3p 5 .
- If there are several subshells, the common shell is omitted: 2s 2 2p 3 becomes 2s 2 p 3 .
- In the case of a further abbreviated notation, the abbreviation of the noble gas with the next lower ordinal number is placed in square brackets and the missing subshells of the element to be displayed are specified.
Example chlorine : 1s 2 2s 2 2p 6 3s 2 3p 5 → [Ne] 3s 2 3p 5 .
The subshells are not to be specified according to the construction principle, but in the order of the main quantum number; so z. B. for europium : [Xe] 4f 7 6s 2 .
In addition, the cell or Pauling notation is common as a clear graphic representation.