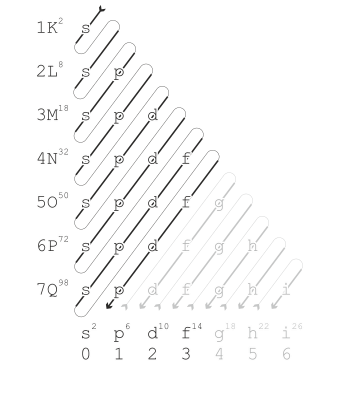Construction principle
The construction principle is a concept developed by Niels Bohr in 1921 in order to be able to explain the periodic appearance of the chemical properties in the periodic table of the elements using the properties of the atomic shell . It is used to determine the arrangement of electrons in atoms , molecules or ions in the lowest energy state. The principle is based on a process that describes the successive filling of the atomic shell with electrons. Electrostatically attracted by the protons in the atomic nucleus , each newly added electron seeks the lowest energy state for itself . This is located in the atomic orbital that has the lowest energy and is not yet fully occupied, whereby the maximum number of electrons in each orbital is given by the Pauli principle . The electron remains in this orbital even if more electrons are added, because the energetic order of the orbitals is almost always retained as the number of electrons increases. Therefore the atomic shells of all atoms have the same structure inside them, only that with increasing nuclear charge the orbitals are bound more tightly and concentrate more closely around the nucleus.
According to the principle, the electrons always fill the orbitals in such a way that the shell adopts the state that has the lowest possible energy within the framework of the model of independent particles. If there are several orbitals with the same energy to choose from for an electron, according to Hund's rules an unoccupied orbital is preferred due to multi-particle effects.
The construction principle can also be used analogously to describe the arrangement of protons and neutrons in the atomic nucleus .
The Madelung Energy Scheme
The order in which the electrons occupy the orbitals is described by the rule (also known as the Madelung rule according to Erwin Madelung or the Klechkowski rule in some mostly French-speaking countries or the Moeller rule in some mostly Spanish-speaking countries) :
- Orbitals with a smaller value are filled before the orbitals with a larger value.
- If the values are the same, the orbital with the smaller value is filled first.
This behavior of the electrons was found out experimentally through the spectroscopic properties of the elements. The quantum mechanical explanation of the rule is based on the total number of nodes in an orbital, which reflects the energy state, as well as on the larger, stronger shielding of the attractive nuclear potential by the other electrons.
Specifically, the electrons are incorporated into the following orbitals one after the other (see Fig. Right):
1s ⇒ 2s ⇒ 2p ⇒ 3s ⇒ 3p ⇒ 4s ⇒ 3d ⇒ 4p ⇒ 5s ⇒ 4d ⇒ 5p ⇒ 6s ⇒ 4f ⇒ 5d ⇒ 6p ⇒ 7s ⇒ 5f ⇒ 6d ⇒ 7p
Note: The Madelung energy scheme can only be applied to neutral atoms in their ground state. (See exceptions)
Explanation of the picture on the right:
The orbitals of the atomic shell are filled with electrons in the order of the arrow. The orbitals of the atomic shell are listed from left to right (increasing secondary quantum number ) and from top to bottom the shells (increasing main quantum number ), each with a letter as the corresponding abbreviation. The superscript numbers indicate the maximum number of electrons that can be in the orbital or in the shell. The value that is decisive for the occupation increases diagonally towards the bottom right in this representation . Therefore, all orbitals on lines perpendicular to this direction each have the same value . According to the rule, in this case the orbitals with the smaller values are first occupied, i.e. H. the individual diagonals are traversed from top right to bottom left. The pale area is of a theoretical nature, as no atoms with so many electrons and the large nuclei that they require have been discovered or created.
exception
The occupation of the shells does not follow the simple structural rule above for all atoms. The reasons are relativistic effects and effects due to the correlations of several electrons with each other, which play an increasingly important role with a larger atomic number, but are not yet taken into account within these structural rules. Examples of elements that behave differently:
- In the case of lanthanum , an electron first occupies an orbital of the 5d subshell before 4f is filled; at Actinium occupied according to an electron 6d before 5f is replenished. The electrons first occupy empty orbitals within a subshell.
- In the case of copper and chromium , an electron of the 4s orbital changes to the 3d orbital, so that the 4s orbital is only occupied once despite its lower energy level. However, the 3d orbitals are half (chrome) or completely (copper) occupied.
The electron configurations beyond the element rutherfordium (atomic number: 104) have not yet been clearly confirmed or proven.
The following periodic table and the following list give an overview of the exceptions, the most noticeable similarities have been summarized.
Extract from the periodic table
| group | 1 | 2 | 3 | 4th | 5 | 6th | 7th | 8th | 9 | 10 | 11 | 12 | 13 | 14th | 15th | 16 | 17th | 18th |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| occupation |
|
d 1 | d 2 | d 3 | d 4 | d 5 | d 6 | d 7 | d 8 | d 9 | d 10 |
|
||||||
| 4th | 19 K |
20 approx |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 mn |
26 feet |
27 Co |
28 Ni |
29 Cu |
30 notes |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 kr |
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mon |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 in |
50 Sn |
51 Sb |
52 te |
53 I. |
54 Xe |
| 6th | 55 Cs |
56 Ba |
57-71 | 72 Hf |
73 days |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 ed |
81 Tl |
82 Pb |
83 bi |
84 Po |
85 at |
86 para |
| 7th | 87 Fr |
88 Ra |
89-103 | 104 para |
105 Db |
106 Sg |
107 hours |
108 ms |
109 m |
110 Ds |
111 Rg |
112 cn |
113 Nh |
114 bottles |
115 Ms |
116 Lv |
117 Ts |
118 above |
|
|
||||||||||||||||||
|
|
occupation | f 1 | f 2 | f 3 | f 4 | f 5 | f 6 | f 7 | f 8 | f 9 | f 10 | f 11 | f 12 | f 13 | f 14 | d 1 | ||
|
|
Lanthanoids | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 pm |
62 Sm |
63 Eu |
64 Gd |
65 p |
66 Dy |
67 Ho |
68 he |
69 Tm |
70 yb |
71 Lu |
||
|
|
Actinoids | 89 Ac |
90 th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 am |
96 cm |
97 Bk |
98 Cf |
99 it |
100 m |
101 Md |
102 No. |
103 Lr |
||
|
|
||||||||||||||||||
| Half-occupied d orbital | No 8 electrons in the d orbital | Electron from s orbital to d orbital | First fill the d orbital | Unknown | ||||||||||||||
| Fully occupied d orbital | No 8 electrons in the f orbital | Electron from the f orbital to the d orbital | other exceptions | no exception | ||||||||||||||
list
| Atomic number | Chemical element | According to the construction principle | Real e-configuration |
|---|---|---|---|
| Half-occupied d orbital | |||
| 24 | chrome | [Ar] 3d 4 4s 2 | [Ar] 3d 5 4s 1 |
| 42 | molybdenum | [Kr] 4d 4 5s 2 | [Kr] 4d 5 5s 1 |
| Fully occupied d orbital | |||
| 29 | copper | [Ar] 3d 9 4s 2 | [Ar] 3d 10 4s 1 |
| 47 | silver | [Kr] 4d 9 5s 2 | [Kr] 4d 10 5s 1 |
| 79 | gold | [Xe] 4f 14 5d 9 6s 2 | [Xe] 4f 14 5d 10 6s 1 |
| No eight electrons in the d orbital | |||
| 28 | nickel | [Ar] 3d 8 4s 2 | [Ar] 3d 9 4s 1 |
| 46 | palladium | [Kr] 4d 8 5s 2 | [Kr] 4d 10 5s 0 |
| 78 | platinum | [Xe] 4f 14 5d 8 6s 2 | [Xe] 4f 14 5d 9 6s 1 |
| No eight electrons in the f orbital | |||
| 64 | Gadolinium | [Xe] 4f 8 6s 2 | [Xe] 4f 7 5d 1 6s 2 |
| 96 | Curium | [Rn] 5f 8 7s 2 | [Rn] 5f 7 6d 1 7s 2 |
| First fill up the empty d orbital | |||
| 57 | Lanthanum | [Xe] 4f 1 6s 2 | [Xe] 5d 1 6s 2 |
| 89 | Actinium | [Rn] 5f 1 7s 2 | [Rn] 6d 1 7s 2 |
| 90 | Thorium | [Rn] 5f 2 7s 2 | [Rn] 6d 2 7s 2 |
| One electron from the s orbital to the d orbital | |||
| 41 | niobium | [Kr] 4d 3 5s 2 | [Kr] 4d 4 5s 1 |
| 44 | Ruthenium | [Kr] 4d 6 5s 2 | [Kr] 4d 7 5s 1 |
| 45 | Rhodium | [Kr] 4d 7 5s 2 | [Kr] 4d 8 5s 1 |
| An electron from the f orbital to the d orbital | |||
| 58 | cerium | [Xe] 4f 2 6s 2 | [Xe] 4f 1 5d 1 6s 2 |
| 91 | Protactinium | [Rn] 5f 3 7s 2 | [Rn] 5f 2 6d 1 7s 2 |
| 92 | uranium | [Rn] 5f 4 7s 2 | [Rn] 5f 3 6d 1 7s 2 |
| 93 | neptunium | [Rn] 5f 5 7s 2 | [Rn] 5f 4 6d 1 7s 2 |
| Other exceptions | |||
| 103 | lawrencium | [Rn] 5f 14 6d 1 7s 2 | [Rn] 5f 14 7s 2 7p 1 |
- ↑ a b With nickel, according to current knowledge, both electron configurations are possible.
- ↑ a b In Lawrencium, quantum mechanical studies suggest a deviation in the electron configuration.
See also
Web links
Individual evidence
- ↑ Niels Bohr: Atomic structure . In: Nature . tape 107 , 1921, pp. 104-107 , doi : 10.1038 / 107104a0 .
- ↑ Eric R. Scerri: How Good Is the Quantum Mechanical Explanation of the Periodic System? . In: Journal of Chemical Education . 75, No. 11, 1998, pp. 1384-85. doi : 10.1021 / ed075p1384 .
- ^ Frank Weinhold, Clark R. Landis: Valency and bonding: A Natural Bond Orbital Donor-Acceptor Perspective. Cambridge University Press, Cambridge 2005, ISBN 0-521-83128-8 , pp. 715-716.
- ↑ Erwin Riedel: Inorganic Chemistry. 2nd Edition. 1990, ISBN 3-11-012321-5 . (for the exceptions to the rule for the occupation of the atomic orbitals)
- ↑ Terry L. Meek, Leland C. Allen ,: Configuration irregularities: deviations from the Madelung rule and inversion of orbital energy levels . In: Chemical Physics Letters . 362, No. 5-6, 2002, pp. 362-364. doi : 10.1016 / S0009-2614 (02) 00919-3 .