Erythritol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
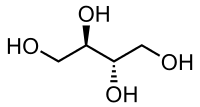
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Erythritol | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 4 H 10 O 4 | |||||||||||||||||||||
| Brief description |
colorless, sweet-tasting crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 122.12 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.45 g cm −3 |
|||||||||||||||||||||
| Melting point |
120-123 ° C |
|||||||||||||||||||||
| boiling point |
329-331 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Erythritol ( meso -1,2,3,4-butanetetrol) is a sweet-tasting compound and chemically belongs to the sugar alcohols . It is the meso form of the optically active Threitol and is used as a sugar substitute . It has about 50–70 percent of the sweetness of sugar ( sucrose ).
Erythritol was discovered by the Scottish chemist John Stenhouse , who isolated the substance as early as 1848. The substance was approved as a food additive without quantity restrictions in the USA in 1997 and in Europe in 2006.
Occurrence
Erythritol occurs naturally in mushrooms, cheese, fruit (strawberries, plums ) or pistachios . For the quantities required by the food industry, erythritol is produced through fermentation .
Extraction and presentation
Erythritol can be produced chemically and catalytically by hydrogenating tartaric acid over Raney nickel catalysts. However, this also creates Threit . Another possibility for production is the conversion of dialdehyde starch into an equimolar mixture of glycol and erythritol. However, since these processes are all very expensive and complicated, erythritol is now produced by microbial conversion of low-molecular carbohydrates (preferably glucose and sucrose ) using osmophilic fungi . Possible by-products of this fermentation are ribitol , glycerine and ethanol as well as lower oligosaccharides.
Effect and side effects
Erythritol contains almost no calories and has no effect on blood sugar and insulin levels . It is approved as a food additive as a sugar substitute in Germany . However, if the proportion exceeds ten percent, it must be marked with a warning.
Compared to other sugar alcohols such as sorbitol , maltitol , lactitol and isomalt, erythritol has the advantage of a particularly high digestive tolerance (approx. 1 g / kg body weight). Since 90 percent of erythritol is absorbed through the small intestine and excreted through the kidneys , the usual side effects of sugar alcohols such as flatulence and diarrhea are greatly reduced, but not entirely excluded.
Since 1990 erythritol has been approved as a food additive in around 60 countries. A content of up to 1.6% in beverages apparently has no laxative effect and an intake of less than 0.78 grams per kilogram of body weight in adults and 0.6 grams in children does not appear to have any undesirable effects.
As a recent study in the journal PNAS showed with 14C-labeled glucose , a small part of glucose is converted into erythritol in humans. If more glucose is absorbed, more erythritol is found in the blood. It remains to be seen whether erythritol is suitable as a biomarker for increased glucose uptake.
According to a study published in June 2014, erythritol is an insecticide that can be used effectively against fruit flies .
Erythritol is also odorless, heat-stable and non-hygroscopic, which means that erythritol does not absorb moisture from the environment, which prevents lump formation. If you dissolve erythritol in water, it has a cooling effect. In addition, it does not promote caries and is used as a sugar substitute for diabetics or people who suffer from fructose intolerance due to its calorie-free properties and long-lasting satiety. In addition, erythritol does not provide a breeding ground for fungi.
Trade names
Sera (D, AT, BX, CZ, FR, IT, RU), Serapur (D, CH, EU), Erylite (D), Sukrin (D, CH), SweetCare natural sweetness (D, CH, EU), Erythritol ( AT, CH, FL, D), Erythritol (D), Neue Süsse (D), Sucolin (D, AT), Xucker Light (D, CH, FL, AT), sweetERY (D), Next Zucker Extra Leicht (AT ), Einser zum Süßen (AT), Eryfly (D, AT), Wiezucker Diet (D, F), Zukka (AT), E968 (alcohol sugar), steviapura Streusweet Plus (D, CH, AT, EU)
Individual evidence
- ↑ Entry on E 968: Erythritol in the European database for food additives, accessed on June 27, 2020.
- ↑ a b c d Entry on erythritol. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2008, ISBN 0-08-094774-3 , pp. 215 ( limited preview in Google Book search).
- ↑ data sheet meso-erythritol from AlfaAesar, accessed on May 14, 2013 ( PDF )(JavaScript required) .
- ↑ a b c data sheet meso-erythritol from Sigma-Aldrich , accessed on May 12, 2017 ( PDF ).
- ↑ Kurt Rosenplenter, U. Nöhle (Ed.): Handbook sweeteners. 2nd edition, Behr's Verlag, 2007, ISBN 978-3-89947-262-2 , p. 431.
- ↑ Erythritol . Zusatzstoffe-online.de, accessed on May 16, 2016.
- ↑ Opinion of the Scientific Committee on Food on Erythritol (PDF; 314 kB). SCF / CS / ADD / EDUL / 215 Final, March 24, 2003.
- ↑ Scientific Panel on Food Additives and Nutrient Sources Added to Food, European Food Safety Authority: Scientific Opinion on the safety of the proposed extension of use of erythritol (E 968) as a food additive . In: EFSA Journal . 13, No. 3, 2015, ISSN 1831-4732 , p. 4033. doi : 10.2903 / j.efsa.2015.4033 . , Quote: "In 2003, the European Union (EU) Scientific Committee on Food (SCF) concluded that erythritol is safe for use in foods. [...] the SCF opinion stated that the laxative threshold may be exceeded, especially by young consumers, [...] the ANS Panel concluded that the acute bolus consumption of erythritol via non-alcoholic beverages at a maximum level of 1.6% would not raise concerns for laxation. "
- ↑ Erythritol - the sweetener that the body produces itself Press release of the TU Braunschweig from May 8, 2017, accessed on July 5, 2018.
- ↑ Kaitlin M. Baudier, Simon D. Kaschock-Marenda, Nirali Patel, Katherine L. Diangelus, Sean O'Donnell, Daniel R. Marenda, Frederic Marion-Poll: Erythritol, a Non-Nutritive Sugar Alcohol Sweetener and the Main Component of Truvia, Is a Palatable Ingested Insecticide. In: PLoS ONE. 9, 2014, p. E98949, doi : 10.1371 / journal.pone.0098949 .
- ↑ Deadly sweetener . Wissenschaft.de, accessed on June 5, 2014.
- ↑ SweetCare natural sweetness. (No longer available online.) In: welzcare.de. Archived from the original on August 30, 2016 ; accessed on August 30, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
