Lactitol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
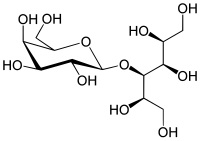
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Lactitol | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 12 H 24 O 11 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Osmotic laxative |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 344.31 g · mol -1 | ||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
> 30,000 mg kg −1 ( LD 50 , rat , oral , lactitol monohydrate) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lactitol ( food additive E 966), also lactitol ( INN ), is a synthetic sugar substitute that does not occur naturally. Lactitol is obtained by catalytic hydrogenation of milk sugar under pressure. The glucose part of the lactose is reduced to sorbitol (glucitol), but the glycosidic bond is not broken. Lactitol is therefore chemically referred to as 4- O- β- D- galactopyranosyl- D -glucitol.
use
sweetener
Lactitol is used as a sweetener for food , especially for diabetics (trade name Lacty ). However, the sweetening power is only about 30-40% that of sucrose , so that its use as a sweetener is limited.
Lactitol only slightly attacks the teeth , but has a relatively strong laxative effect and is therefore also used for this purpose. Long and intensive studies have shown that lactitol has no toxicologically important side effects.
Therapeutically
Lactitol is used therapeutically for the symptomatic treatment of constipation , which cannot be improved by other measures, and for the symptomatic treatment of hepatic encephalopathy .
The effect is based on the conversion of lactitol into short-chain organic acids, which increase the osmotic pressure in the colon and consequently the fluid influx, which is responsible for the laxative effect (trade name Importal ).
Individual evidence
- ^ A b c The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; ISBN 978-0-911910-00-1
- ↑ Data sheet D-Lactitol monohydrate from Sigma-Aldrich , accessed on November 8, 2016 ( PDF ).
- ↑ LACTITOL MONOHYDRATE CRS data sheet (PDF) at EDQM , accessed on June 24, 2009.
- ↑ L. O'Brien Nabors (Ed.): Alternative Sweeteners . Marcel Dekker Inc., 3rd exp. Edition 2001, ISBN 0-8247-0437-1 , pp. 297-299.
- ↑ a b c Entry on lactitol. In: Römpp Online . Georg Thieme Verlag, accessed on October 1, 2014.
- ^ Alfred Larry Branen, P. Michael Davidson and Branen Larry Branen: Food Additives . Marcel Dekker Inc; 2nd edition 2001; ISBN 978-0-8247-9343-2 ; Pp. 466-467.