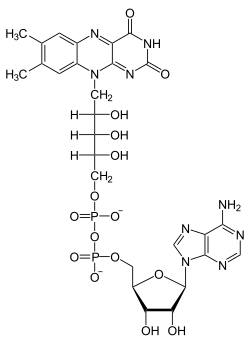
Flavin adenine dinucleotide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Flavin adenine dinucleotide | |||||||||||||||||||||
| Molecular formula | C 27 H 33 N 9 O 15 P 2 | |||||||||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 785.55 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| solubility |
soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Flavin adenine dinucleotide , or FAD for short, is a coenzyme . It has an important role as an electron carrier in various prokaryotic and eukaryotic metabolic processes , such as oxidative phosphorylation , β-oxidation of fatty acids , the respiratory chain and other redox reactions . In contrast to NAD +, FAD can transfer individual electrons . Oxidoreductases can thus activate molecular oxygen by means of FAD .
Structure and chemical properties
FAD consists of adenosine diphosphate linked to riboflavin ( vitamin B 2 ). Alternatively, one could also say that it consists of adenosine monophosphate (AMP) to which flavin mononucleotide (FMN) is bound. The “reactive” nitrogen atoms are located in the isoalloxazine ring of the molecule.
The oxidized FAD changes into the reduced form FADH 2 by taking up two protons (H + ) and two electrons (e - ) : This is known as an ECEC mechanism ( e for electrochemical step, c for chemical step of protonation), where the second protonation takes place only in a sufficiently acidic solution. The transition between the ECE mechanism without a final transfer of a proton and the ECEC mechanism is also dependent on the chemical environment: For free FAD in solution, the ECE and ECEC mechanisms overlap at pH 6.7; FAD that is immobilized on surfaces only becomes reduced at about pH 9 according to the ECE mechanism.
The redox potential of the FAD under standard conditions is −219 mV vs. NHE.
A solution of flavin adenine dinucleotide in water (10 g l −1 ) has a pH value of about 6.
Enzymes that use FAD
The enzymes that use FAD include the:
- Monoamine oxidase
- Ferredoxin-NADP + reductase
- Glucose oxidase (GOx)
- Cellobiose dehydrogenase
- Nitrate reductase
- Succinate dehydrogenase
- Dihydrolipoyl dehydrogenase
- Acyl-CoA dehydrogenase
See also
Web links
Individual evidence
- ↑ a b c Entry on flavin adenine dinucleotide. In: Römpp Online . Georg Thieme Verlag, accessed on February 13, 2019.
- ↑ a b data sheet Flavin adenine dinucleotide disodium salt hydrate from Sigma-Aldrich , accessed on February 13, 2019 ( PDF ).
- ↑ U. Dettmer, M. Folkerts, E. Kächler, A. Sönnichsen: Intensive course in biochemistry , 1st edition, Elsevier Verlag, Munich 2005, ISBN 3-437-44450-6 , p. 10.
- ^ A b K. Aktories, U. Förstermann, FB Hofmann, K. Starke: General and Special Pharmacology and Toxicology: Founded by W. Forth, D. Henschler, W. Rummel , 10th edition, Elsevier Verlag, Munich, ISBN 3 -437-42522-6 , p. 762.
- ^ H. Renz: Integrative Clinical Chemistry and Laboratory Medicine. Pathophysiology - Pathobiochemistry - Haematalogy , 1st edition, de Gruyter Verlag, Berlin 2003, ISBN 3-11-017367-0 , p. 616.
- ↑ a b Müller, F .; Chemistry and Biochemistry of Flavoenzymes, 1991, Vol. 1, CRC Press London.
- ↑ Nöll et al., In: Langmuir B, 2006, 22, pp. 2378-2383.
