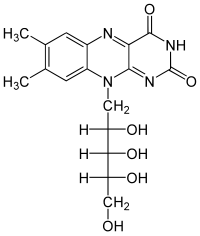
Riboflavin
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
| General | |||||||||
| Common name | Vitamin B 2 | ||||||||
| other names | |||||||||
| Molecular formula | C 17 H 20 N 4 O 6 | ||||||||
| CAS number | 83-88-5 | ||||||||
| PubChem | 1072 | ||||||||
| ATC code | |||||||||
| DrugBank | DB00140 | ||||||||
| Brief description | bitter-tasting, yellow to orange-colored solid | ||||||||
| Occurrence | Liver, yeast, wheat germ | ||||||||
| physiology | |||||||||
| function | Flavin - Coenzyme ( FAD , FMN ) precursor | ||||||||
| Daily need | 1.5-1.7 mg | ||||||||
| Consequences in case of deficiency | Inflammation of the skin ( exanthema , skin cracks), disorders of growth, blood formation and neurological nature | ||||||||
| Overdose | not known | ||||||||
| properties | |||||||||
| Molar mass | 376.37 g mol −1 | ||||||||
| Physical state | firmly | ||||||||
| Melting point |
278–282 ° C (decomposition) |
||||||||
| solubility |
|
||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Riboflavin , also lactoflavin or vitamin B 2 , formerly called vitamin G , is a vitamin from the B complex . It is also known colloquially as the growth vitamin.
history
Vitamin B 2 was first isolated from milk (lacto) in 1920 and contains a yellow chromophore (flavin) and a ribityl residue (ribo).
Occurrence
Vitamin B 2 can be found in food as unbound riboflavin or bound to proteins. It occurs in milk and dairy products , among other things , but also in vegetables such as broccoli , asparagus or spinach , as well as in fish, muscle meat, eggs and whole grain products .
It has been produced biotechnologically since 1990 with the help of the filamentous fungus Ashbya gossypii . The wild type produces up to 100 mg riboflavin per g biomass, the production strains supply more than 20 g / L. Alternatively, riboflavin is also produced by genetically modified strains of Bacillus subtilis .
properties
Riboflavin is a derivative of the heterocycle pteridine , more precisely the isoalloxazine and the sugar alcohol ribitol . Riboflavin is one of the water-soluble vitamins , although it is not very soluble in water . it is light-sensitive, but so stable to heat and oxygen that it is not destroyed when cooked.
function
Riboflavin serves as a precursor for flavin - coenzymes ( FAD , FMN ), which in particular oxidoreductases , z. B. NADH dehydrogenase , play a major role. As a result, it plays a central role in the metabolism .
The alleged recommendation of neurologists for a tablet of 100 mg or 400 mg riboflavin per day for prophylaxis against migraines contrasts with the assessment of an S3 guideline that there is an indication, but not a proof of the effectiveness of riboflavin (vitamin B2) Migraine prophylaxis can be talked about .
Daily requirement
The daily requirement is around 1.5 mg and is usually covered by normal food intake.
- Infants: 0.3-0.4 mg
- Children and adolescents (up to 15 years): 0.7–1.6 mg
- Adolescents (from 15 years) and adults: 1.2–1.5 mg
- Pregnant women: 1.5 mg
- Breastfeeding: 1.6 mg
Deficiency symptoms
With a normal diet, there are no deficiency symptoms. However, deficiency symptoms can occur in pregnant women and alcoholics , which manifest themselves in exanthema , cracks in the skin (especially on the lips or in the corner of the mouth, cheilosis ) and sensitivity to light. This hypovitaminosis is called ariboflavinosis or B 2 avitaminosis . The EGRAC can be determined for the early detection of a riboflavin deficiency .
In migraine research it is assumed that migraine patients may suffer from an undersupply of the brain metabolism with riboflavin, which can be remedied or alleviated by adding more vitamins.
Overdose
Overdoses are not known in humans. The toxicity of B 2 is very low. As with other water-soluble vitamins , riboflavin that is not required or that can be absorbed by the body is usually excreted in the urine or stool.
Other use
The water-soluble, heat-stable and light-sensitive riboflavin is used as a yellow food color (E101).
It is also often used to control cleaning processes (surfaces, hands, etc.) in the pharmaceutical industry, as it glows in low concentrations with UV light and is therefore clearly visible.
Individual evidence
- ↑ Entry on E 101: Riboflavins in the European database on food additives, accessed on June 16, 2020.
- ↑ a b c d e f g h i j Entry on riboflavin. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ↑ a b c d Entry on riboflavin in the GESTIS substance database of the IFA , accessed on June 25, 2017(JavaScript required) .
- ^ D. Steinhilber, M. Schubert-Zsilavecz, HJ Roth: Medicinal Chemistry - Targets and Drugs. Deutscher Apotheker Verlag, Stuttgart 2005, ISBN 3-7692-3483-9 .
- ↑ H. Sahm, G. Antanikian, KP. Stahmann, R. Takors: Industrial Microbiology . 1st edition. Springer Spectrum, Berlin / Heidelberg 2013, ISBN 978-3-8274-3039-7 .
- ↑ J. Schoenen, J. Jacquy, M. Lenaerts: Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. In: Neurology. Volume 50, Number 2, February 1998, pp. 466-470, PMID 9484373 .
- ↑ C. Boehnke, U. Reuter, U. Flach, S. Schuh-Hofer, KM Einhäupl, G. Arnold: High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care center. In: European journal of neurology. Volume 11, Number 7, July 2004, pp. 475-477, doi : 10.1111 / j.1468-1331.2004.00813.x , PMID 15257686 .
- ↑ G. Haag, H.-C. Diener, A. May, C. Meyer, H. Morck, A. Straube, P. Wessely, S. Evers: Self-medication for migraine and tension-type headache. Evidence-based recommendations from the German Migraine and Headache Society (DMKG), the German Society for Neurology (DGN), the Austrian Headache Society (ÖKSG) and the Swiss Headache Society (SKG). (PDF; 341 kB). German Migraine and Headache Society , S3 guideline, Schattauer-Verlag, 2009, p. 394.
- ↑ Ludwig Weissbecker: B 2 -avitaminosis (ariboflavinosis). In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid. 1961, p. 1092 f.
Web links
- HA Zappe among others: Riboflavin deficiency in Baltistan (Little Tibet). ( Memento from December 2, 2012 in the web archive archive.today ) Annual meeting of the German Tropical Medicine Society, Heidelberg, 1997.
