Malolactic fermentation
| Parent |
|
Fermentation metabolism of L- lactic acid |
| Gene Ontology |
|---|
| QuickGO |
The malolactic fermentation occurs in many heterofermentative lactic acid bacteria and yeasts and plays in the wine , fruit juice and champagne manufacturing a role. It is also known as malolactic fermentation (BSA) or malic acid fermentation. The malolactic fermentation is a secondary fermentation, which results in the degradation of acid in the medium. It follows a primary, alcohol-producing fermentation. During malolactic fermentation, certain lactic acid bacteria decarboxylate the dicarboxylic acid L - malate (= deprotonated form of L - malic acid ) while generating energy into the weaker monocarboxylic acid L - lactate (= deprotonated form of L - lactic acid ). The lactic acid is further broken down into ethanol and fermented by the baker's yeast Saccharomyces cerevisiae .
The malolactic fermentation resulting from this fermentation leads to a harmonious and more balanced taste in the winemaking process - an effect that increases quality, but which also harbors risks: low-acid wines are less durable, less “tangy” and do not appear as fresh. Malolactic fermentation that is carried out too far can also lead to an annoying aftertaste reminiscent of milk or cheese.
Occurrence
Malolactic fermentation takes place in many microorganisms that use heterofermentative fermentation to generate energy. Oenococcus oeni (old name Leuconostoc oenos ) is important for winemaking, as is Lactobacillus spp. (e.g. Lactobacillus plantarum ) and Pediococcus spp.
Also yeasts such as Saccharomyces cerevisiae can at the end of alcoholic fermentation run malolactic fermentation.
biochemistry
process
| before [g · l −1 ] |
afterwards [g · l −1 ] |
|
| Malic acid | 3.2 | 0.5 |
| Lactic acid | 0.12 | 1.8 |
| Total acidity | 4.9 | 3.8 |
During malolactic fermentation, the decarboxylation of L- malate to L- lactate is catalyzed. Depending on the pH of the medium, malate or lactate can also be protonated
In this reaction, with every gram of degraded malic acid, the total acid content in the wine is reduced by 0.4 g / L H 2 SO 4 .
S. cerevisiae breaks down malate into lactate via an NADH-dependent malate enzyme. This creates NADH. Lactate is eventually decarboxylated to ethanal , which is then reduced to ethanol . The reduction requires NADH. Strictly speaking, therefore, one can only speak of fermentation when malate is broken down in yeast, while bacteria decarboxylate it.
Energy generation
Under standard conditions , energy is released in the decarboxylation reaction. This value depends on the pH value. At pH 7, 26.5 kJ mol −1 are released , at a pH value of 5.7 33.9 kJ mol −1 are released. In wines, the pH value can be around 3.5, which would release 46.5 kJ · mol −1 under standard conditions . However, it should be noted that under physiological conditions malate, lactate and CO 2 are not present in a concentration of 1 mol·l −1 and that malate is constantly consumed and lactate is produced during the process. Therefore, the energy gain is lower under physiological conditions.
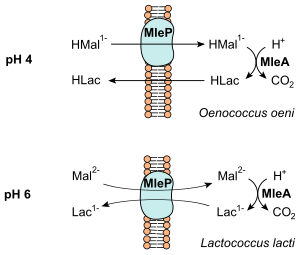
The energy released during decarboxylation is conserved in the form of a chemiosmotic mechanism (see figure). In O. oeni , which grows at a pH of around 4, protonated malate (HMal 1− ) is simply brought into the cell by a membrane-based carrier (MleP). This is decarboxylated to lactate, which is protonated (HLac) at pH 4. The outflow from the cell at HLac takes place without a carrier. Lactococcus lactis grows under higher pH values and malate (Mal 2− ) is exchanged for lactate (Lac 1− ) by an antiporter (MleP).
In both cases a net (negative) charge is transported, which leads to an energization of the membrane. In addition, a proton is consumed in the course of the decarboxylation, so that a difference in proton concentration can also be built up. Both processes therefore lead to a build-up of an electrochemical gradient ( proton motive force ). It is used by the bacteria to maintain the pH value and to absorb nutrients. However, the energy released during malolactic fermentation is not sufficient as the only energy source, so that the heterofermentative lactic acid bacteria continue to rely on the fermentation of pentoses or hexoses .
history
At the end of the 19th century, Alfred Koch proved that bacteria are responsible for reducing acidity in wines. These findings were published in 1900 during a conference of the German Viticulture Congress in Colmar . Further findings, e.g. B. the negative effects of bacterial acid degradation, summarized in 1913 Hermann Müller (Thurgau) and Adolf Osterwalder in a publication.
Although the chemical reaction had been known since the early 1920s through the work of Wenzel Seifert at the Higher Federal College and Federal Office for Viticulture and Fruit Growing in Klosterneuburg , it took almost 40 years to gain a deeper understanding of malolactic acid degradation.
Paul Kulisch and Julius Wortmann suggested that malate degradation can also be caused by yeast . J. Schukow provided proof of this at the Geisenheim research institute . However, this fact was not paid attention to until many years later and was taken up again in the work of Radle in the 1990s.
literature
- Jancis Robinson : The Oxford Wine Lexicon . 1st edition. Gräfe and Unzer Verlag, Munich 2003, ISBN 3-7742-0914-6 .
- Helmut H. Dittrich, Manfred Grossmann: Microbiology of Wine . 3., rework. Edition. Ulmer Eugen Verlag, Stuttgart 2005, ISBN 3-8001-4470-0 .
Individual evidence
- ↑ a b T. Zaunmüller et al .: Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. In: Appl Microbiol Biotechnol. 72 (3), 2006, pp. 421-429. PMID 16826375 ; doi: 10.1007 / s00253-006-0514-3 .
- ↑ S. Kolb et al: Energy conservation in malolactic fermentation by Lactobacillus plantarum and Lactobacillus sake. In: Arch Microbiol. 157 (5), 1992, pp. 457-463. PMID 1510572 . doi: 10.1007 / BF00249105 .
- ↑ EB Olsen et al .: Electrogenic L-malate transport by Lactobacillus plantarum: a basis for energy derivation from malolactic fermentation. In: J Bacteriol. 173 (19), 1991, pp. 6199-6206. PMID 1917854 ; PMC 208371 (free full text).
- ^ A b S. Q. Liu: A review: malolactic fermentation in wine - beyond deacidification. In: J Appl Microbiol. 92 (4), 2002, pp. 589-601. PMID 11966898 .
- ↑ A. Koch: About the causes of the disappearance of the acid during fermentation and storage of the wine. 1900.
- ↑ Hermann Müller-Thurgau, A. Osterwalder: The bacteria in wine and fruit wine and the changes caused by them. In: Centralblatt for Bacteriology, Parasite Science and Infectious Diseases. Dept. 2, Volume 36, Jena 1913, pp. 129-338.
- ↑ L. Alzinger, R. Eder: Influence of various yeast preparations on the acid composition of wines of the Grüner Veltliner variety. In: Mitt. Klosterneuburg. 53, 2003, pp. 52-60.