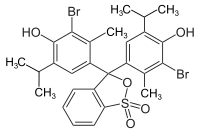
Bromothymol blue
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Bromothymol blue | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 27 H 28 Br 2 O 5 S | |||||||||||||||
| Brief description |
pink powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 624.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
200-202 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bromthymol blue is a triphenylmethane dye and belongs to the group of sulfonphthaleins . It is accessible by bromination of thymol blue . The sodium salt is a pH indicator .
properties
Bromothymol blue changes from yellow to blue at a pH value of 5.8–7.6. Neutral solutions (pH = 7.0) are colored green. His pK s value is 7.1. The color change is based on a change in the chemical structure. In an acidic medium, the molecule is in the pale yellow sulton structure . A ring opening with formation of the intensely blue colored, quinoid triphenylmethane structure takes place in a basic medium.
use
Bromthymol blue can be used as an indicator in acid-base titration and to determine the neutral point (pH = 7.0) in titration , as the color changes to green at 7.0. This is used, for example, as an indicator for the CO 2 content of an aquarium. The desired value of the CO 2 supply can be set via the typical color change .
Furthermore, bromothymol blue is used as a dye in bacteriological culture media and as a sensitizer in electrophotography .
In obstetrics Bromthymolblau is used for the diagnosis of membrane rupture.
Individual evidence
- ↑ a b c Entry on bromothymol blue. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c Datasheet Bromothymol Blue, ACS reagent, Dye content 95% from Sigma-Aldrich , accessed on December 1, 2019 ( PDF ).
- ^ Ricardo E. Felberbaum: Specialist examination in gynecology and obstetrics. Elsevier, 2005 ( limited preview in Google Book Search).

