Brombull valenes
Brombullvalene are chemical compounds which are derived from C 10 H 10 - hydrocarbon bullvalene derived and one or more bromine substituents have. Bromobull valenes with up to six bromine atoms are known. Like the parent compound Bullvalen, most Brombullvalene also show the phenomenon of rapid valence isomerization . Each of the ten carbon atoms can occupy any position ( bridgehead , cyclopropyl position , and olefinic position adjacent to the bridgehead atom or the cyclopropane ring) and be directly connected to any other carbon atom. The transition between the different valence isomers occurs through Cope rearrangements .
presentation
By bromination of Bullvalen 1 with elemental bromine , a 1,4-addition gives the bicyclic dibromide 2 . The subsequent dehydrohalogenation with a strong base, for example potassium hydroxide , potassium tert -butanolate , diazabicyclonones (DBN) or diazabicycloundecene (DBU) results in the mixture of several monobromobullvalues 3 . It is a transannular 1,4-elimination with regression of the bullvalene structure.
By repeating this synthesis sequence several times, the more highly substituted bromobullvalene derivatives di-, tri-, tetra-, penta and hexabromobullvalene are accessible.
properties
Brombullvalen
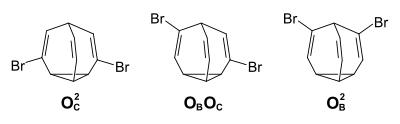
While there are 1.2 million structurally identical valence isomers in the unsubstituted bullvalene (“fluctuating structure”), these are no longer identical in the monosubstituted bullvalene. The substituent can occupy the bridgehead position B (120 960 valence isomers), a position on the cyclopropane ring C (362 880 valence isomers) or an olefinic position adjacent to the bridgehead O B , or adjacent to the cyclopropane ring O C (362 880 valence isomers in each case).
-

possible structural isomers of brombullvalene
(B: bridgehead position, C: cyclopran position, O B : olefinic position adjacent to the bridgehead,
O C : olefinic position adjacent to the cyclopropane ring)
The temperature-dependent 1 H- NMR spectra show that the two olefinic positional isomers are preferred with a proportion of 50% each in the equilibrium mixture.
Dibromobullvalene
Dibromobullvalene also shows the phenomenon of rapid valence isomerization. Fifteen different isomers are possible, nine achiral positional isomers and three pairs of enantiomers . In the equilibrium mixture, only the purely olefinic isomers with the bromine substituents on various ethene units are present (O B O C enantiomers 59%, O C O C 27%, O B O B 14%).
Tribromobullvalene
In the bullvalene derivatives with three identical substituents, the number of possible isomers increases to 46-3 isomers with a C 3v symmetry (10,080 degenerate valence isomers each), 13 isomers with a C 2v symmetry (each 30,240 degenerate valence isomers), and 26 isomers with no symmetry element (60,480 degenerate valence isomers for each of the 13 enantiomer pairs). In the case of tribromobullvalene, of the 29 different possible positional isomers in the equilibrium mixture, only the purely olefinic positional isomers with one substituent on each ethene bridge are present (O B O C O C 48%, O B O B O C 32%, O C O C O C 14%, O B O B O B 6%)
Tetrabrombullvalen
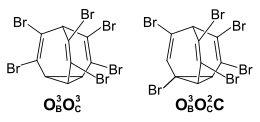
With a further bromine substituent, the number of possible positional isomers in tetrabromobullvalene increases to 47, with 25 positional isomers having no element of symmetry and thus being present as pairs of enantiomers (a total of 72 distinguishable isomers). The 1 H-NMR spectrum at −20 ° C shows that in equilibrium, in addition to the three exclusively olefinically substituted positional isomers (pair of enantiomers O B O B O C O C 49%, O B O C O C O C 23%, O B O B O B O C 8%) about 20% positional isomers with a bromine atom on the cycloprpane ring and three olefinic bromine substituents are also present.
The increase in temperature in the 1 H-NMR spectrum leads to a broadening of the band, as in the case of the lower substituted bromobull valenas, but the signals are not completely mixed because the sample decomposes at an increased temperature.
The 1 H-NMR spectrum of a freshly dissolved sample of crystallized tetrabromobullvalene shows that this is almost isomerically pure (O B O C O C O C isomer). It follows from this that no isomerization processes take place in the crystal and the establishment of equilibrium only takes place in solution. At 10 ° C, the concentration of the isomer is halved in one hour. The O B O B O B O C can be isolated from the equilibrium mixture of tetrabromobullvalene by chromatography . The establishment of equilibrium starting from this isomer takes place much more slowly. At 40 ° C, the concentration halves in 90 minutes.
Pentabrombullvalen
The maximum number of positional isomers possible is achieved with pentabromobullvalene. There are 84 different isomers (24 positional isomers with C 2v symmetry and 30 pairs of enation meres), of which the two positional isomers with exclusively olefinic substitution (54% and 24%) and two positional isomers with one cyclopopyl and three olefinic substituents (13 % and 9%) are available.
The isomer with three O B and two O C substituents (24% in the equilibrium mixture) can be isolated by chromatography. It is a kinetically very stable isomer that does not isomerize even after several days in solution at room temperature. The half-life of equilibrium at 55 ° C is approx. 30 hours.
Hexabrombullvalen
The number of possible isomers of Hexabrombullvalen corresponds to that of Tetrabrombullvalen. Two positional isomers can be isolated - the purely olefinically substituted isomer and the isomer with a doubly substituted ethene unit and an O B C substitution on the third bridge.
Even after several days at 60 ° C., the 1 H-NMR spectrum of the isomer with the cyclopropyl substituent shows no proportions of the purely olefinically substituted isomer, while about 10% of this has been converted into the other isomer after 10 days. In the case of Hexabrombullval, the typical bullval characteristic of the fluctuating structure has largely been lost.
use
Starting from the Brombullvalenes, Bullvalene derivatives with other substituents can be prepared with halogen exchange reactions. Among other things, Bullvalene derivatives with methyl , phenyl , fluoro and hydroxymethyl substituents are known, it being possible for these to be identical or different if there are several substituents.
About the Dihydroxymethylbullvalen are by reaction with polyethylene glycol - ditosylates under basic conditions different crown ether accessible, the Bullvalene typical temperature dependence of 1 show H-NMR spectra. Due to the different possible positional isomers, the ring size of the crown ethers is variable. The term “breathing” crown ethers was coined for this class of substances.
-

Bullvaleno crown ether
n = 1: Bullvaleno [11-13] crown-3 n = 2: Bullvaleno [14-16] crown-4
n = 3: Bullvaleno [17-19] crown-5 n = 4: Bullvaleno [20- 22] crown-6
n = 5: Bullvaleno [23-25] crown-7
Individual evidence
- ↑ G. Schröder , R. Merényi, JFM Oth: Molecules with rapid and reversible valence isomerization . II . In: Tetrahedron Letters . tape 5 , no. January 14 , 1964, p. 773-777 , doi : 10.1016 / 0040-4039 (64) 83034-3 .
- ↑ JFM Oth, R. Merényi, G. Engel and G. Schröder: Synthesis and NMR spectroscopic behavior of two disubstituted bull valenes. In: Tetrahedron Letters . tape 7 , no. January 29 , 1966, p. 3377-3382 , doi : 10.1016 / s0040-4039 (01) 82797-0 .
- ^ A b Bernhard Volkmann, Gerhard Schröder: Isolable positional isomers of Tetrabrombullvalens . In: Chemical Reports . tape 117 , no. 6 , June 1984, pp. 2226-2232 , doi : 10.1002 / cber.19841170615 .
- ^ A b c Karl Rebsamen, Gerhard Schröder: Penta- and Hexabrombullvalene . In: Chemical Reports . tape 126 , no. 6 , June 1993, p. 1425-1427 , doi : 10.1002 / cber.19931260623 .
- ^ Jean FM Oth, Robert Merényi, Jan Nielsen, Gerhard Schröder: Molecules with rapid and reversible valence isomerization, VI. Syntheses and properties of some monosubstituted bull valenes . In: Chemical Reports . tape 98 , no. October 10 , 1965, p. 3385-3400 , doi : 10.1002 / cber.19650981040 .
- ↑ a b c Karl Rebsamen: Methyl- and Phenylbullvalene . Dissertation, University of Karlsruhe (TH). Karlsruhe 1986.
- ↑ Keshab Sarma, Walter Witt, Gerhard Schröder: On the question of positional isomerism in disubstituted bull valenes . In: Chemical Reports . tape 119 , no. 7 , July 1986, pp. 2339-2349 , doi : 10.1002 / cber.19861190724 .
- ↑ Keshab Sarma, Walter Witt, Gerhard Schröder: Bullvaleno-crown ether - crown ether with variable ring sizes . In: Chemical Reports . tape 116 , no. December 12 , 1983, p. 3800-3812 , doi : 10.1002 / cber.19831161205 .