3,4-homotropilids
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | 3,4-homotropilids | |||||||||
| other names |
Bicyclo [5.1.0] octa-2,5-dienes |
|||||||||
| Molecular formula | C 8 H 10 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 106.16 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
3,4-homotropilidene is an unsaturated bicyclic hydrocarbon in which a 1,4-cycloheptadiene unit having a cyclopropane ring fused is.
presentation
3,4-Homotropiliden is obtained by cyclopropanation of cycloheptatriene ( tropiliden ). According to a process by Gaspar and Roth , tropilides 1 is reacted with gaseous diazomethane in the presence of copper (I) chloride as a catalyst . A mixture of 3,4-homotropilids 2 and 1,2-homotropilids 3 is obtained , which can be separated by gas chromatography .
Synthesis of homotropilids by cyclopropanization of tropilids
properties
Thermal rearrangement at high temperature
While 1,2-homotropilids rearrange at 225 ° C., 3,4-homotropilids are surprisingly stable. Tetrahydropentalen is only formed at a temperature of 305 ° C:
Thermal rearrangement of 3,4-homotropilids
to tetrahydropentals
Degenerate Cope rearrangement
The most striking property of the 3,4-homotropilid can be seen in the temperature dependence of the NMR spectrum . At low temperatures (−50 ° C) the spectrum is well resolved with a pronounced fine structure for the signals of the four cyclopropyl and vinyl H atoms or the two aliphatic H atoms. When warming to room temperature, the spectrum becomes more diffuse and of the ten hydrogen atoms only the vinyl hydrogen atoms can be clearly assigned. At 180 ° C, a new spectrum is finally formed in which the signals for the vinyl H atoms only occupy half the area and the cyclopropyl H atom signals have completely disappeared. This observation can be traced back to a degenerate Cope rearrangement :
Degenerate Cope rearrangement
in 3,4-homotropilidae
At high temperatures, the hydrogen atoms swap their place so quickly that the mean values of the signals can be observed in the NMR spectrum. The term “fluctuating structure” was coined for this behavior.
3,4-homotropilids can adopt both a transoid conformation ( 1 ) and a cisoid conformation ( 2 ). The transoid conformation is thermodynamically favored, but much less favorable for the Cope rearrangement compared to the cisoid conformation. The Cope rearrangement ( B ) in the 3,4-homotropilids is therefore preceded by a conformational reversal ( A ) from the cis to the trans conformation. After the Cope rearrangement, the molecule is again in the cis conformation and is again in equilibrium with the trans conformation:
Homotropilids:
A ) equilibrium between trans-conformation 1 and cis-conformation 2
B ) degenerate Cope rearrangement.
It can be estimated that a 3,4-homotropilid molecule undergoes a Cope rearrangement about a thousand times per second at 180 ° C and about once per second at −50 ° C.
Compounds with 3,8-homotropilid structural element
If the cis conformation of the 3,4-homotropilid structure is enforced by the incorporation of a bridge between the cyclopropyl and the aliphatic position, the Cope rearrangement also proceeds significantly faster. Examples of other compounds with a fluctuating structure are the Barbaralan , Barbaralon and Semibullvalen :
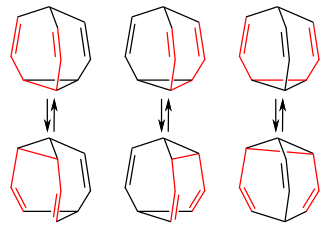
If the 3,4-homotropilid is bridged with a further double compound, the result is bullvalene , a highly symmetrical molecule with three 3,4-homotropilid units. Successive degenerate Cope rearrangements result in around 1.2 million valence isomers , in which each of the ten carbon atoms can occupy any position in the molecule and can be linked to any other carbon atom:
Bullvalene with three Cope systems (red) of a bullvalene molecule
and with the respective valence isomers of the corresponding Cope rearrangement
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ W. von E. Doering, WR Roth: A rapidly reversible degenerate cope rearrangement . In: Tetrahedron . tape 19 , no. 5 , January 1963, p. 715 , doi : 10.1016 / S0040-4020 (01) 99207-5 .
- ^ A b W. VE Doering, WR Roth: Thermal rearrangement reactions . In: Angewandte Chemie . tape 75 , no. 1 , January 7, 1963, p. 27 , doi : 10.1002 / anie.19630750106 .
- ↑ Sebastian Ehrhart, Martin Empting, Dominik Ruppert: Barbaralon. (PDF) Retrieved October 1, 2019 .