Nitronium ion
The nitronium ion (or nitryl cation ) NO 2 + is a cation that is unstable under normal conditions and is formed by removing an electron from paramagnetic nitrogen dioxide , or by protonation and subsequent elimination of water from nitric acid .
Despite its instability, it is used in many ways in the electrophilic substitution of other, in particular organic compounds , especially in the nitration of aromatics . The ion is formed in situ by mixing nitric acid and sulfuric acid . The following reaction takes place:
The nitronium ion still exists in solid form as dinitrogen pentoxide , N 2 O 5 , an ionic solid consisting of nitronium and nitrate ions . However, the liquid or gaseous forms are always molecular and therefore do not contain any nitronium ions. Some nitronium salts with anions of low nucleophilicity , such as nitronium tetrafluoroborate or nitronium perchlorate (NO 2 + ClO 4 - ) can be isolated, but are very reactive.
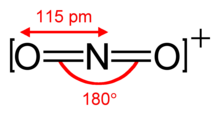
The nitronium ion is isoelectronic to carbon dioxide and also has the linear structure with an ONO bond angle of 180 °.
Related species
The compounds of nitryl fluoride , NO 2 F, and nitryl chloride , NO 2 Cl, are not nitronium salts, but molecular compounds such as the low boiling point (-72 ° C or -6 ° C), and the short nitrogen - halogen - bond lengths show (NF 135 pm, N-Cl 184 pm).
The addition of an electron creates a neutral nitryl radical : • NO 2 - a stable and well-known compound: nitrogen dioxide. This is in a temperature and pressure dependent equilibrium with its dimer , dinitrogen tetroxide (N 2 O 4 ) . N 2 O 4 is not a radical.
The associated negatively charged species is NO 2 - , the nitrite ion .
Individual evidence
- ^ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, pp. 220-221, ISBN 3-211-81060-9 .
- ↑ FACotton and G. Wilkinson: Advanced Inorganic Chemistry . 5th edition (1988), Wiley, p. 333.