ionization
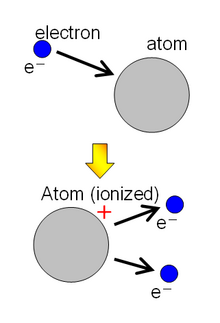
Ionization is any process in which one or more electrons are removed from an atom or molecule so that the atom or molecule remains as a positively charged ion ( cation ). The reverse process, in which an electron is captured by a positively charged atom or molecule, is called recombination .
Another form of ionization, which is particularly relevant in chemistry, is the attachment of electrons to a neutral atom or molecule, so that a negatively charged ion ( anion ) is created. Also, chemical ionization effected by addition of ions (protons, cations, anions), for example. B. in mass spectrometry .
If the nucleus of an atom is pushed out of the electron shell - e.g. B. by a fast neutron - it also becomes an ion. However, the term ionization is not common for this process.
In the literature, there are also formulations such as "acids that give weakly nucleophilic anions when ionized - such. B. HSO 4 - from H 2 SO 4 - can be broken down into […]. ” In a broader sense, the elimination of a proton in an acid-base reaction can also be subsumed under the term ionization.
Mechanisms
Various processes can lead to ionization:
- Ionizing radiation (including, for example, accelerated electrons in a thyratron tube) can " knock out" electrons from their bond through impact ionization . The released electrons can in turn ionize further if they have enough energy. At a sufficiently high temperature, an electron, ion or neutral atom can also cause impact ionization without additional acceleration of particles due to its disordered temperature movement.
- In field ionization , electrons are released from their bond by a sufficiently strong electric field .
- Highly excited atoms can automatically change into an ionized state through autoionization . Field ionization is essentially a process of autoionization, i.e. That is, a highly excited atom or molecule spontaneously loses an electron without any further interaction with the energy source.
Symbolic spellings
To describe the impact ionization process , symbols such as (e, 2e), (e, 3e), (γ, 2e) etc. are often used - analogous to the notation in nuclear reactions . The first character in brackets stands for the projectile. After the decimal point are the free particles produced (in addition to the ionized atom and including the projectile, provided that it is not absorbed - as in the case of the photon ). For example, “2e” means that two free electrons leave the atom. In (e, 2e) a single ionized atom is produced by the collision of an electron with an atom, in (γ, 2e) a doubly ionized atom is produced by the interaction of a photon with an atom.
Ionization energies

For all ionization processes, energy must be applied to separate the electron from the atom or molecule ( ionization energy ). In the previous section, possible sources for this energy were mentioned. Ionization energies are typically on the order of several electron volts (example argon in the ground state: 15.7 eV). Ionization energies depend on the material to be ionized and its current state of excitation. It is becoming increasingly difficult to further ionize atoms or molecules that have already been ionized. The ionization energy increases exponentially with each electron to be removed from the electron shell.
plasma
Plasma is matter with a sufficiently high proportion of free ions and electrons, i.e. high ionization. This qualitative definition is not limited to gases with a low density, but also includes compressed matter with the properties of a liquid. In the case of gases, a distinction is made between low , atmospheric and high pressure plasmas . Almost all of the visible matter in the universe is more or less strongly ionized.
Application examples

Air that is ionized by means of ionizers , i.e. electrically conductive air, is used in the processing of products that can become electrostatically charged, e.g. B. Foil or paper rolls. The conductivity of the air reduces the charge and thus eliminates the risk of sparks and the attraction of unwanted dust particles. Transport is also made easier.
Level of ion content in natural and indoor environments:
- In close proximity to waterfalls 20,000–70,000 ions / cm³
- In the mountains or near the sea, 4,000–10,000 ions / cm³
- On the outskirts, on meadows and in fields 1,000–3,000 ions / cm³
- Inner-city parks 400–600 ions / cm³
- In the city and agglomeration 200–500 ions / cm³
- In ventilated or air-conditioned rooms 10–100 ions / cm³ "
These ion concentrations are measured with an ionometer. Here the polarity and the respective concentration of the ions can be determined. In nature, the ratio of the natural polarity of the ions is usually balanced, with a slight tendency towards more positively charged ions. The ion concentration depends on the geological composition, the geographical location and the weather conditions.
Ionized air is used, for example, in the food industry for the pasteurization of beer and other beverages. In beverage filling, the bottle is blown out with ionized air before filling begins in order to kill microorganisms.
Ionizing radiation is used in industrial sterilization (e.g. of single-use medical items, to kill insects, inactivation of enzymes). In hospitals which has plasma sterilization , the gas sterilization largely replaced.
Harmful effects
In some cases , the generation of ions in direct or indirect ionization creates radicals that lead to chemical reactions and, among other things, the formation of ozone , nitrogen oxides and other pollutants. Ozone can affect the human respiratory system and promote corrosion . The direct ionization (mainly of water molecules) in the human body by radiation leads to the formation of H + and OH - radicals, which attack organic molecules.
See also
- Ionizing , ionisation , ion beam radiation , ionizing radiation , ion source , ionizer
- Autoionization
Web links
- Ionization energy database
- ionization
- Solutions for industry: ionization and suction, article in electronics practice: "Barrier-free ESD protection in new dimensions"
Individual evidence
- ^ A b Wolfgang Karl Ernst Finkelnburg: Introduction to Atomic Physics . Springer-Verlag, 2013, ISBN 978-3-662-28827-6 , pp. 20 ( limited preview in Google Book search).
- ↑ a b c d Jürgen H. Gross: mass spectrometry - a textbook . Springer-Verlag, 2012, ISBN 978-3-8274-2981-0 , pp. 384 ( limited preview in Google Book Search).
- ↑ P. Sykes: How do organic reactions work ?: Reaction mechanisms for beginners. Wiley-VCH Verlag, 2001, p. 89.
- ↑ Ulrich Stroth: Plasma Physics Phenomena, Basics, Applications . Springer-Verlag, 2011, ISBN 978-3-8348-8326-1 , p. 2 ( limited preview in Google Book search).
- ↑ Lucerne University of Applied Sciences and Arts - ionized air in the interior (PDF file), issued in January 2013, p. 19, accessed June 6, 2013.
- ↑ Heinz M. Hiersig : Lexikon production technology process technology . Springer-Verlag, 2013, ISBN 978-3-642-57851-9 , pp. 85 ( limited preview in Google Book search).
- ^ Rainer Klischies, Ursula Panther, Vera Singbeil-Grischkat: Hygiene and medical microbiology. Textbook for nursing professions; with 62 tables . Schattauer Verlag, 2008, ISBN 978-3-7945-2542-3 , pp. 207 ( limited preview in Google Book search).
- ^ Holger Luczak: Ergonomics . Springer-Verlag, 2013, ISBN 978-3-662-05831-2 , pp. 328 ( limited preview in Google Book search).
- ^ Thomas J. Vogl, Wolfgang Reith, Ernst J. Rummeny: Diagnostic and interventional radiology . Springer-Verlag, 2011, ISBN 978-3-540-87668-7 , pp. 12 ( limited preview in Google Book search).
