Radical theory
The radical theory was understood as a theory developed by Justus von Liebig , Friedrich Wöhler and Auguste Laurent around 1830 on the structure of organic compounds .
Auguste Laurent then also used the term radical for parent and secondary nuclei from individual atoms or groups of atoms in nuclear theory .
The term radical in terms of radical theory is not to be equated with the modern term radical in current theoretical chemical concepts. In the radical theory, statements are made about the composition of a chemical body that consists of several small groups of elements, namely the radicals.
Together with the new methods of elemental analysis, also developed by Justus von Liebig, the theory pioneered the understanding of organic chemistry and its reactions in the 19th century.
Other contemporaneous considerations were the substitution theory drafted in 1834 (see history of the substitution reaction ) by Jean-Baptiste Dumas with the idea of the substitutability of hydrogen in organic compounds, e.g. B. by halogens.
Problem
When examining many compounds, most of which were isolated from natural substances , it turned out that they very often only consist of three or four elements , namely carbon , hydrogen , oxygen and, less often, nitrogen . With the help of elemental analysis, the molecular formula of the substances could be determined, but even then a great number of substances were known that had completely different properties with the same composition (molecular formula). The chemical properties also depended on the connection between the elements, but the structure of a compound could not be determined with the earlier methods.
In addition, it was assumed at that time that in a chemical reaction all substances involved are broken down into their constituent parts and reassembled. However, this contradicted the fact that the molecular formula of the substances often only changes slightly during a reaction and the findings from the inorganic chemistry that has already been further developed could therefore not be applied.
Radical composition model
Liebig and Wöhler therefore developed a model in which they assumed that a substance consists of several smaller groups of elements, the so-called radicals , that would be taken over unchanged in a reaction. The term radical is only to be understood in the sense of a group of atoms , since the modern atomic models did not yet exist in Liebig's time .
The model can be clearly compared with a prefabricated house. If the owner of the house wants a bay window instead of a balcony, there is no point in tearing down the entire house and rebuilding it together with the fabric of the bay window. Instead, only the old balcony will be chipped off and the bay window will be replaced.
Attempts to confirm the theory
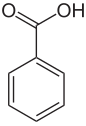
| Benzoic acid (C 7 H 6 O 2 ) | Benzaldehyde (C 7 H 6 O) | Benzyl alcohol (C 7 H 8 O) |
To confirm the theory, Liebig and Wöhler investigated the composition of benzoin , a drug obtained from the resin of an East Indian plant, but which could also be obtained by leaving bitter almond oil, a well-known and popular fragrance, in the air. Today the active ingredient from benzoin is known as benzoic acid , while the main component of bitter almond oil is benzaldehyde . They found that the two structures in the empirical formula differed only in one oxygen atom. In addition, they were able to produce another substance through chemical reactions, which they called benzoic substance (today benzyl alcohol ), in which the hydrogen content was two units higher than in bitter almond oil.
After the fundamental relationship of the three substances had been proven, they carried out various chemical reactions with the substances, including the reaction with chlorine , bromine , iodine and ammonia . They were able to show that a basic structure with the formula unit C 7 H 5 O always remained unaffected in the reactions . Liebig and Wöhler called this basic structure benzoyl and viewed it as a radical of benzoic acid that is surrounded by all other substances.
“ We find that they are all grouped around a single compound which, in almost all of its union relationships with other bodies, does not change its nature or its composition. This constancy, this consistency in the phenomena, led us to accept this compound as a compound raw material and to propose a special name for it, the name Benzoyl. "
Effects of radical theory
Radical theory can be seen as one of the cornerstones of modern organic chemistry, as it was here that the concept of dividing a molecule into several functional units was described for the first time. This concept is still used today in the context of the functional groups and is the basis of the division into substance classes. The systematic nomenclature of compounds also uses these subdivisions, with the C 7 H 5 O unit based on benzaldehyde still being given the prefix benzoyl- .
literature
- Johannes Valentin: Friedrich Wöhler: Great natural scientist , Dr. Frickhinger, HW Volume 7. Stuttgart: Wissenschaftliche Verlagsgesellschaft MBH, 1949, DNB 455202664 .
Web links
- Section in the memorandum ( memento of March 28, 2007 in the Internet Archive ) by Emil Erlenmeyer on Liebig's work
- modern chemistry . In: Meyers Konversations-Lexikon . 4th edition. Volume 3, Verlag des Bibliographisches Institut, Leipzig / Vienna 1885–1892, p. 985.
Individual evidence
- ↑ Edvard Hjelt: History of organic chemistry from the earliest times to the present . Vieweg + Teubner, Wiesbaden 1916, ISBN 978-3-663-07241-6 , doi : 10.1007 / 978-3-663-07241-6 .