List of atomic models

An atomic model is an idea of the structure and shape of atoms . Even in ancient times there was the atomic hypothesis , according to which the atoms were regarded as the indivisible and unchangeable basic building blocks of all material substances. The atomic hypothesis could initially only be based on the philosophical preference for a particle model over the hypothesis of the infinitely continuable divisibility of matter. The different properties of material substances should also be attributed to the possible combinations of a few types of atoms. It was not until the beginning of the 19th century that scientific evidence of the real existence of atoms became apparent in chemistry and physics : The atom was defined as the smallest unit of a chemical element , and the behavior of gases, according to the kinetic gas theory, could be completely derived from disordered movement a large number of identical molecules , each consisting of a few atoms, can be explained. As an atomic model, the idea of a small sphere with a diameter of approx. 0.1 nm and a mass of approx. 10 −26 kg was sufficient . In this form, the atomic hypothesis had largely prevailed at the end of the 19th century, when new observations with electron beams and radioactive substances showed that these atoms themselves consist of smaller particles. The explanation of their complicated internal structure led to quantum mechanics in 1925 , whose atomic models are primarily formulated as mathematical statements. When asked how one should imagine an atom, Werner Heisenberg , one of the creators of quantum mechanics , replied : "Don't even try!"
The following, chronologically ordered list gives an overview. Major models have main articles. Current models are also shown in context in the article Atom .
- The particle model of Democritus (around 400 BC) postulates the existence of different types of solid, indivisible particles which, when combined in different ways, form the known substances.
- The Dalton model (1803) is based on the smallest, indivisible particles, which differ in mass depending on the element and are linked to each other in different substances in certain number ratios (depending on the type of substance). When the substances change through chemical reactions, the atoms can only rearrange themselves.
- In the dynamide model (1903), atoms consist of small, rotating electrical dipoles, the dynamides, and the empty space between them.
- According to Thomson's atomic model (1903), the atom consists of an evenly distributed positive charge and negatively charged electrons that move around in it. This model is also known as the plum pudding model or raisin cake model in German . It can explain why the atoms are permeable to high-energy particle beams (such as cathode rays , alpha rays ), because the positive charge is assumed to be free of matter.
- In the planetary model or Saturn model by Nagaoka Hantarō (1904), the atom consists of a positively charged sphere, which is orbited by the negatively charged electrons. In analogy to the stability of Saturn's rings , the model correctly postulates a very massive nucleus, but incorrectly also postulates energy radiation through the movement of the electrons.
- In Haas' atomic model from 1910, a quantum condition is introduced for the first time . The hydrogen atom should consist of a homogeneously charged positive body (as with Thomson), on the surface of which an electron rotates in a circle. Haas determines the size by identifying - without further explanation - the energy of this state (potential calculated from the rest position in the center) with the energy of a photon at the short-wave limit of the Balmer series . The same formula results as later in Bohr's atomic model for the size of the first excited state, which is also the basic state of the Balmer series.
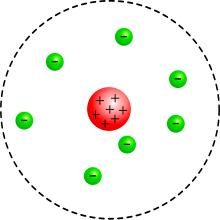
- According to Rutherford's atomic model (1911), the atom consists of a very small positively charged atomic nucleus, which contains almost the entire mass of the atom, and which is surrounded by an atomic shell of electrons in a manner not described further. This could explain the observation of the rare strong deflections of alpha particles.
- According to Bohr's model of the atom (1913), the atom consists of a positively charged, mass-bearing nucleus and electrons that circle around it in certain orbits without emitting energy. With the help of this model, some energy levels of the hydrogen atoms could be calculated with good accuracy for the first time.
- The Bohr-Sommerfeld atomic model (1916) is an extension of Bohr's atomic model, in which certain elliptical orbits around the atomic nucleus are also permitted.
- The shell model (atomic physics) simplifies the atom in such a way that a positively charged atomic nucleus is surrounded by spherical shells in which the electrons are located. Only the outermost shell is responsible for the chemical properties of the element. No statement is made about the movement of the electrons.
- According to the orbital model (1928), the atom consists of a nucleus surrounded by electrons in orbitals. The shape of the orbitals is given by the spatial probability of the electrons being present. In the strict sense, an orbital is a stationary solution of the quantum mechanical Schrödinger equation (an energy state of an electron).
- The ball cloud model ( kimballsches atomic model , tetrahedral model ) is a simple extension of the shell model in the direction of the orbital model. It is used more often in school.
- In the Thomas-Fermi model (1928), the electron shell is described across the board as a Fermi gas that is enclosed in the potential well that results from the electrical attraction of the electrons by the nucleus.
- In some areas, atoms can still be approximated as rigid bodies: Either as points without expansion, as in the kinetic gas theory in the model of the ideal gas , or as spheres with a certain volume and attractive forces, as in the Van der Waals gas . The corresponding model is also called the point particle model or the incompressible sphere model .
Models of the atomic nucleus
- The droplet model (1936) describes the atomic nucleus as droplets in an electrically charged liquid.
- The shell model (1949) describes the atomic nucleus in close analogy to the orbital model of the atomic shell.
literature
- Károly Simonyi: The cultural history of physics . Harri Deutsch, Thun, Frankfurt am Main 1995, ISBN 3-8171-1379-X .
- Helge Kragh: Before Bohr: Theories of atomic structure 1850-1913. (= Research Publications on Science Studies. RePoSS 10). Department of Science Studies, University of Aarhus Oct. 2010. (ivs.au.dk)
- Philipp Bohr: Physics. Textbook for the advanced level . 2006, ISBN 3-8334-5041-X .
Web links
Individual evidence
- ↑ Dieter B. Herrmann : Big Bang in the Laboratory: How Particle Accelerators Simulate Nature . Springer, 2010, ISBN 978-3-642-10314-8 , pp. 36 .