Spherical cloud model
The spherical cloud model ( KWM , also Kimball's atomic model and tetrahedron model ) is an atomic model that can be used to illustrate some phenomena - such as covalent bonds or molecular structure . It represents an extension of Bohr's atomic model and is a simplification compared to the more precise orbital model . The model goes back to the American quantum chemist George Elbert Kimball (1906–1967).
The electron shells that are arranged around the atomic nucleus are taken from Bohr's shell model . In each shell, due to the Pauli principle , a maximum of two electrons are combined in a spherical cloud (spherical electron cloud ). The electron cloud is to be understood as a “living space” for the electrons in which they move.
The further away an electron shell is from the center of the atom, the more electron clouds can be geometrically arranged around the nucleus. Accordingly, with increasing distance, more and more electron clouds fit on a shell:
- First shell (K shell) = 2 electrons = 1 electron cloud
- Second shell (L shell) = 8 electrons = 4 electron clouds
- Third shell (M-shell) = 18 electrons = 9 electron clouds
- Fourth shell (N shell) = 32 electrons = 16 electron clouds
The number of electrons in the n have space th shell, calculated according to the formula
- Maximum number of electrons = 2 • n 2 .
However, as in Bohr's model, each outer shell does not accept more than 8 electrons.
Rules for filling up the electron clouds
- In the first shell there is only one spherical cloud, which is arranged centrally around the core.
- From the second shell onwards, 4 electron clouds are always created, only in the subgroups they are expanded to the final number (although this does not matter in the chemical bond )
- Each of the four electron clouds is initially simply occupied due to the repulsion of the electrons. Only from the 5th electron on the shell are the electrons distributed in pairs in the electron clouds.
- The electron clouds always try to achieve the greatest possible distance, which is why they are arranged in a tetrahedral manner.
- The occupation of the inner shells is not taken into account because they play no role in the chemical bond.
- The further occupation of the electron clouds and shells takes place according to the Madelung energy scheme .
Examples
Hydrogen atom
The first shell has only one spherical cloud, which is arranged centrally around the core.Oxygen atom
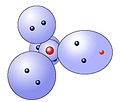
In the second shell there are four spherical clouds that have to take 6 electrons together for oxygen.Hydrochloric molecule
The binding occurs by overlapping the singly occupied electron clouds from the hydrogen and chlorine materialize
The following graphic shows the elements of the 2nd period in valence notation. Fully occupied spherical clouds are represented by a line and half-occupied by a point.
Web links
- short introduction to the state education server BW
- Further considerations and visualizations on the quantitative Lewis model, which is based on Kimball's spherical clouds by Ernst Schumacher (English)
Individual evidence
- ↑ Elements Chemistry I - Baden-Württemberg , 2004, pp. 162–165.