Transition State Theory
The theory of the transition state ( English Transition state theory , TST), also transition state theory called (after Henry Eyring , 1901-1981), is a molecular theory of reaction kinetics . It was derived taking into account molecular quantities, the sum of states , and enables the determination of the absolute reaction rate constants of a chemical reaction . This reaction rate can then be used in a rate equation . In addition to Eyring in Princeton, Michael Polanyi and Meredith Gwynne Evans in England were among the founders of the theory .
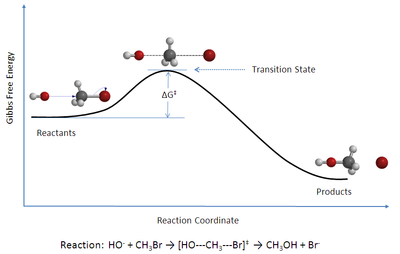
The starting materials are separated from the products by a potential wall (activation barrier), which represents a saddle point on the potential hypersurface. The reaction of the educts via the transition state to the products runs along a trajectory , the reaction coordinate (path between the educts and products with a minimal change in potential energy in each case). The point of highest potential energy on this reaction coordinate is the transition state . The term activated complex describes the arrangement of the particles in the transition state (it is not a complex in the sense of the article complex chemistry ). Kramer's theory of reaction kinetics extends the TST.
The main assumptions on which the TST is based are:
- Separation of nucleus and electron movement, analogous to the Born-Oppenheimer approximation
- The probability density of the energy states of the educts can be described by a Boltzmann distribution .
- In the transition state, the movement along the reaction coordinate can be separated from other movements and treated classically as a translation .
- The transition state is in equilibrium with the educts . (Quasi-equilibrium hypothesis)
- Only educts react to products, not the other way around (one-way traffic).
The result of the derivation is the Eyring equation :
= Rate constant , = transmission coefficient (not in the sense of optics), = Boltzmann constant , = temperature, = Planck's constant of action , = equilibrium constant of the transition state.
The TST explains the mechanism of catalysis : by lowering the activation energy, the reaction rate constant is increased.
Derivation
The derivation is made for an example reaction in which the starting materials and the product react. The transition state is defined as an intermediate stage .
The reaction rate is defined as the rate of product formation,
where the relative activity of the transition state is given by the equilibrium constant of the preceding equilibrium (according to the reaction quotient )
and the concentrations of A and B are replaced. It results:
One summarizes and relates the product formation rate to the educts and
and gets for the rate constant
The further derivation differs depending on the textbook. The result is the Eyring equation given above.
The rate constant results as
whereby the transmission coefficient is not derived, but is introduced as an additional parameter for adapting experimental results to the calculated ones.
So the following applies:
- .
with and the difference between the standard free energies of the educts and the transition state.
has a value of and is referred to as the frequency factor. It is in the order of magnitude of the collision frequencies of the molecules in liquids .
This gives the final form:
Thermodynamic formulation
With the van-'t-Hoff reaction isobar we get:
The Legendre transformation of the Gibbs-Helmholtz equation allows a representation as:
The Arrhenius equation gives a formal definition for the activation energy :
The Eyring equation can be rewritten analogously, taking into account the van-'t-Hoff reaction isobars :
With the definition of enthalpy (at constant pressure) it follows:
For unimolecular reactions and for reactions in solutions and condensed matter is approximately zero:
For ideal gases , the pre-exponential factor is:
criticism
- The TST is based on the classic mechanics . In reactions of very light species, for example hydrogen or deuterium atoms, tunnel effects occur, for the description of which a quantum mechanical TST would be necessary. This problem has not yet been solved satisfactorily.
- Only if the movement on the potential energy surface is a one-dimensional movement along the reaction coordinates is the assumption justified that the movement along the reaction coordinates can be separated from the other degrees of freedom . But this assumption is necessary for the derivation of the Eyring equation.
- For higher temperatures, anharmonic corrections of the potential at the saddle point are necessary.
See also
Web links
- Theory of the transition state: Consideration from phase space ( Memento from April 24, 2009 in the Internet Archive ) (English, PDF)
Individual evidence
- ↑ Entry on Transition State Theory . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.T06470 Version: 2.3.3.
- ^ H. Eyring: The Activated Complex in Chemical Reactions . In: J. Chem. Phys. 1935 , 3 , 107; doi : 10.1063 / 1.1749604 .
- ↑ Entry on Activated Complex . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00092 Version: 2.3.3.












![{\ displaystyle {\ frac {\ mathrm {d} \ mathrm {[P]}} {\ mathrm {d} t}} = k ^ {\ ddagger} \ cdot \ mathrm {[C ^ {\ ddagger}]} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/abb6d7eacc55bc4cd924648b5ca9f7f115584d00)
![\ mathrm {[C ^ {\ ddagger}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a0f99bec1afeb7632554e0efa3914547efa27bbb)
![{\ displaystyle K ^ {\ ddagger} = \ mathrm {\ frac {[C ^ {\ ddagger}]} {[A] \ cdot [B]}} = \ exp (- \ Delta {G ^ {\ ddagger} } ^ {\ circ} / (k _ {\ mathrm {B}} T))}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c2ed15184e66f719025b728d53697445bfea622d)
![{\ displaystyle {\ frac {\ mathrm {d} \ mathrm {[P]}} {\ mathrm {d} t}} = k ^ {\ ddagger} \ cdot K ^ {\ ddagger} \ mathrm {\ cdot [ A] \ cdot [B]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2edaaf3f76b1b2e3a15df884f954603eca9e8de4)


![{\ displaystyle {\ frac {\ mathrm {d} \ mathrm {[P]}} {\ mathrm {d} t}} = k \ mathrm {\ cdot [A] \ cdot [B]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7de0656eaf5779f3583dbe5f4965b6ac7a59ccd9)























