Cobalamins
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|

|
|||||||||
|
Coenzyme B 12 (= AdoCbl): R = 5′- deoxyadenosyl cyanocobalamin (= vitamin B 12 ): R = –C≡N aquocobalamin (= vitamin B 12a ): R = –OH 2 hydroxycobalamin (= vitamin B 12b ): R = -OH methylcobalamin (= MeCbl or MeB 12 ): R = -CH 3 |
|||||||||
| General | |||||||||
| Common name | Vitamin B12 | ||||||||
| other names |
|
||||||||
| Molecular formula |
|
||||||||
| CAS number |
|
||||||||
| PubChem | 16072210 | ||||||||
| ATC code | |||||||||
| DrugBank | DB00115 | ||||||||
| Brief description | red, crystalline solid (cyanocobalamin, hydroxocobalamin, methylcobalamin) | ||||||||
| Occurrence | animal products (adenosylcobalamin, hydroxocobalamin, methylcobalamin), large intestine (through bacterial production) | ||||||||
| physiology | |||||||||
| function | Cell division, blood formation, function of the nervous system | ||||||||
| Daily need | 4 µg | ||||||||
| Consequences in case of deficiency | Pernicious anemia , neurological diseases (e.g. funicular myelosis ), glossitis , diarrhea | ||||||||
| Overdose | not known | ||||||||
| properties | |||||||||
| Molar mass | |||||||||
| Physical state | firmly | ||||||||
| Melting point |
decomposes above 392 ° C (cyanocobalamin) |
||||||||
| solubility | Slightly soluble in water: 12 g l −1 (cyanocobalamin), 20 g l −1 (hydroxocobalamin), insoluble in ether, acetone and chloroform (cyanocobalamin), soluble in alcohol (cyanocobalamin) | ||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Cobalamins are chemical compounds that occur in all living things and are also referred to as the vitamin B 12 group (simply vitamin B 12 ). The most important member of the cobalamin group is coenzyme B 12 , which is also known colloquially as vitamin B 12 and , as a cofactor ( coenzyme ), is part of several enzymes . Two cobalamin-dependent enzymes are known to be involved in the metabolism of amino acids in humans . Cobalamins contain the trace element cobalt as a central atom.
Storage forms also belong to the vitamin B 12 group
- Aquocobalamin or Aquacobalamin (vitamin B 12a , the conjugate acid of hydroxo cobalamin),
- Hydroxocobalamin or hydroxycobalamin (vitamin B 12b ) and
- Nitritocobalamin (vitamin B 12c ).
The medicine uses the biologically inactive forms cyanocobalamin and hydroxocobalamin for vitamin B 12 - Supplementation and for other therapeutic purposes. In the human organism, these precursors are converted into adenosylcobalamin and methylcobalamin . Both are biologically active as coenzymes. Adenosylcobalamin (AdoCbl) is also known as extrinsic factor (English extrinsic factor ).
Definitions
The lexicon of medical laboratory diagnostics defines vitamin B 12 as “water-soluble vitamins which, in the form of two coenzymes, play an essential role in the cellular metabolism of fatty acids and aliphatic amino acids ”. Accordingly, the term substituted corrinoids or cobalamins with biological effects are summarized. Synonymous terms of vitamin B 12 based on research history are antiperniziosa factor and extrinisic factor . The definition of the Pschyrembel emphasizes the involvement of the vitamin group in cell division , erythropoiesis and myelin formation .
The International Union of Nutritional Sciences (IUNS) and the International Union of Pure and Applied Chemistry (IUAPC) state that "Vitamin B 12 " should be used as a general description of any corrinoids that qualitatively exhibit the biological activity of cyanocobalamin.
In PubChem , vitamin B 12 is synonymous with cyanocobalamin, as is the case in various specialist literature.
Not to be confused with the actual cobalamins are the trans- cobalamins , which are only transport proteins for vitamin B 12 .
history
After the American pathologist George H. Whipple had discovered in the early 1920s that dogs suffering from pernicious anemia ( malignant anemia ) could be cured of this otherwise fatal disease by feeding them raw liver, the search led to the essential component of this healing method finally in 1926 for the description of an anti-pernicious factor that is also effective in humans by the two American doctors George R. Minot and William P. Murphy , who together with Whipple received the Nobel Prize for Medicine in 1934.
The active ingredient was only isolated in crystalline form in 1948, independently of one another, both by a team of American biochemists led by Karl A. Folkers ( Merck & Co. ) and a British research team led by chemist E. Lester Smith ( Glaxo ). The red crystalline compound was called "Vitamin B 12 ". In the same year, vitamin B 12 was found in milk powder , in beef extract and liquid cultures of various types of bacteria .
In 1955, the British biochemist Dorothy C. Hodgkin was able to elucidate the molecular structure of cyanocobalamin single crystals with the help of X-ray diffraction , for which she u. a. 1964 was honored with the Nobel Prize for Chemistry.
Around 1956, it was believed that vitamin B 12 had been found in cyanocobalamin , which, in view of its use in effective vitamin preparations, contributed to the confusion of terms, even though it is actually a biologically inactive form.
The total synthesis of cyanocobalamin based on this, then known as "vitamin B 12 ", was achieved by Albert Eschenmoser and Robert B. Woodward in 1972 and this vitamin B 12 form is still considered one of the largest molecules ever to be fully synthesized in a laboratory.
description
Cobalamins are organometallic compounds with a central one, two or three times positively charged cobalt - Ion and as such the so far only known cobalt-containing natural products . Naturally occurring cobalamins with vitamin B 12 effects are adenosylcobalamin , methylcobalamin and hydroxocobalamin . Cyanocobalamin , on the other hand, is the industrially produced, stable form of cobalamin that does not occur in nature.
All cobalamins have the same basic structure of a cobalt complex , in which the cobalt cation consists of five nitrogen atoms and a sixth, i. d. Usually interchangeable ligand is surrounded, see adjacent figure. Four of the nitrogen atoms belong to a flat corrin ring system (marked in blue), which surrounds the cobalt cation so tightly that it can only be released again by destroying the ring system, while the fifth nitrogen atom is a nucleotide -like 5,6-dimethylbenzimidazole bonded to the corrin ring -Ring heard.
The name given to the respective cobalamin is the sixth, interchangeable ligand, which is usually abbreviated to R (for remainder) in the chemical structural formulas: If R is a hydroxyl group , the cobalamin is hydroxy cobalamin, R is a cyano group , cyano cobalamin, and at a 5 ' Desoxyadenosylliganden as a residual to the 5'-deoxyadenosyl cobalamin, short coenzyme B 12 .
Usually the sixth ligand R is only weakly bound to the cobalt cation, so that it can easily be exchanged for other ligands and the human organism, for example, the therapeutically used hydroxy cobalamin or cyano cobalamin (vitamin B 12 ) by exchanging the hydroxy for a cyano group and this in turn can convert against a 5'-deoxyadenosyl group into the actually biochemically active coenzyme B 12 .
Cyano cobalamin (vitamin B 12 ) itself is an odorless, deep, dark red, crystalline, hygroscopic substance that dissolves only moderately in water and lower alcohols , but not at all in (non-polar) organic solvents such as acetone , chloroform or ether .
In the slightly acidic pH range of 4–6, vitamin B 12 is quite temperature stable. However, greater losses can occur in basic solutions or in the presence of reducing agents such as ascorbic acid (vitamin C) and SO 2 . The half-life of vitamin B 12 at a pH value of 5.5 and 30 times as much vitamin C in the dark was determined to be less than 15 hours (classic mineral water has pH 5.5 and multivitamin tablets sometimes contain 32,000 times as much vitamin C as B 12 ).
In the cell, cobalamins are mainly found in the cytosol as methyl cobalamin, in the mitochondria, on the other hand, mainly as 5'-deoxyadenosyl cobalamin (coenzyme B 12 ). The figure above shows the general structural formula of the cobalamins with R as the exchangeable ligand.
Function in the organism
To put it simply, vitamin B 12 is important for cell division and blood formation as well as the function of the nervous system .
Coenzyme B 12 takes part in only two enzymatic reactions in the human organism as a coenzyme :
- N 5 -methyl tetrahydrofolate homocysteine S -methyl transferase ( methionine synthase , EC 2.1.1.13 ) and
- Methylmalonyl-CoA mutase ( EC 5.4.99.2 )
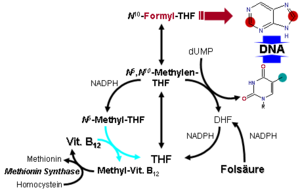
The methionine synthase reaction serves u. a. the regeneration of the methyl group carrier S -adenosylmethionine (SAM) or the formation of methionine. Here is homocysteine remethylated to methionine. If this does not succeed, more homocysteine is formed, an intermediate product in the breakdown of the amino acid methionine (increased homocysteine levels are associated with the formation of arteriosclerosis ). As a methyl group while acting N 5 -methyl-tetrahydrofolate ( N 5 -methyl- THF ). If coenzyme B 12 is missing , N 5 -methyl-THF accumulates and there is a secondary deficiency in THF, which is necessary for the synthesis of the purine bases adenine and guanine and the pyrimidine base thymine . A lack of these nucleobases disrupts the synthesis of DNA in particular, but also RNA . This manifests itself primarily in organs with high cell division activity such as the bone marrow . There is a more or less pronounced pancytopenia in the blood, the deficiency of erythrocytes - anemia - being the most obvious. The remaining erythrocytes become so enriched with hemoglobin that they have a higher hemoglobin content than normal erythrocytes. These cells are also somewhat larger. Therefore one speaks of a hyperchromic , macrocytic anemia. This block can be circumvented by administering folic acid , but this approach does not solve the underlying vitamin B 12 deficiency, so that the treatment of pernicious (literally dangerous) or megaloblastic anemia with vitamin B 12 deficiency with folic acid is a malpractice represents.
The reason for this is the additional function of vitamin B 12 in methylmalonyl-CoA mutase. This is used to smuggle the terminal propionyl-CoAs of odd-numbered fatty acids, as well as parts of the carbon structure of the amino acids valine , isoleucine , threonine and methionine into the mitochondrial citric acid cycle . The methylmalonyl-CoA formed during the breakdown of these compounds from propionyl-CoA (in a biotin- dependent step) is converted by the vitamin B 12 -dependent methylmalonyl-CoA mutase to succinyl-CoA , an intermediate product of the citrate cycle.
If this step is inhibited, there is an increase in methylmalonic acid in the plasma and especially in the urine. This metabolic pathway apparently plays a special role in the CNS , as a vitamin B 12 deficiency sometimes even occurs before the typical anemia with symptoms such as B. funicular myelosis , a disorder of the pyramidal tract and the posterior cords , but also apparent senile dementia and other noticeable. Therefore, especially in older patients with neurological symptoms, a vitamin B 12 deficiency should be excluded as a possible (co-) cause and treated if necessary. The first neurological symptoms are expressed as so-called polyneuropathy in the form of tingling paresthesia or other abnormal sensations (e.g. slight burning sensation) in various parts of the body, which are initially only temporary.
Occurrence
| Food | Content in µg / 100 g (see note) |
|---|---|
| Beef liver | 65 |
| Calf liver | 60 |
| Spirulina maxima | (57) |
| Pork liver | 40 |
| Veal kidney | 25th |
| Chicken liver | 20th |
| Pig kidney | 15th |
| Oysters | 14.6 |
| herring | 11 |
| mackerel | 9 |
| Beef (muscle) | 5.0 |
| tuna | 4.3 |
| Camembert 30% fat i. Tr. | 3.1 |
| salmon | 2.9 |
| Steppe cricket | 2.9 |
| Emmentaler 45% fat i. Tr. | 2.2 |
| Chicken egg yolk | 2.0 |
| eel | 1 |
| Blood sausage | 1 |
| Pork (muscle) | 0.8 |
| Quark 20% fat i. Tr. | 0.8 |
| cod | 0.5-0.8 |
| Cow's milk 3.5% fat | 0.4 |
| soy sauce | 0.3 |
| Tempeh | 0.3 |
| ginger | 0.16 |
| Egg white | 0.1 |
| vegetables | 0.01 |
| sauerkraut | 0 |
Cobalamin sources
Animals are not able to produce vitamin B 12 themselves. Vitamin B 12 is naturally produced by microorganisms - especially bacteria - which occur as symbionts both in the digestive tract of animals and on the surface of plant hosts (e.g. legumes ).
In the human and animal organism, vitamin B 12 is mainly accumulated in the liver and kidneys , in plants it can only occur in traces. In higher plants, vitamin B 12 is mainly found in the roots, but almost not in the green parts.
Omnivores and carnivores cover their B 12 needs by consuming meat , especially offal . Poultry species and pigs can only insufficiently absorb the B 12 produced in their intestines and are therefore dependent on an exogenous supply through their feed. In the pig to the feed for this purpose either is fish meal or vitamin B 12 is added; the latter in a dosage of 10 µg per kg feed. Bacteria that produce vitamin B 12 are also found in the human colon . However, this synthesis is not sufficient to meet the needs and the vitamin formed there is mainly excreted with the stool. The vitamin B 12 supplied with food is absorbed by the enterocytes . This cell type is particularly common in the small intestinal epithelium .
In ruminants , the vitamin is formed in the forestomach , in other herbivores in the large intestine . A deficiency in ruminants is usually due to insufficient cobalt intake. In animal production , traces of cobalt are added to the feed if the animals have to be fed on pastures that are poor in cobalt . This is intended to counteract growth and lactation disorders , anemia and loss of appetite . Some types of herbivores can supply vitamin B 12 from ingesting their appendix feces . The excrement is rich in vitamins of the B complex and vitamin K . The behavior is called cecotrophy and is known from rodents (Rodentia) and rabbits (Lagomorpha) , among others . Preventing rabbits from ingesting their appendix feces reduces the utilization of nutrients, including vitamins, from the feed.
Cobalamine content of some foods
Note on the table : The information does not correspond to the content of the vitamin in the food that the human organism can use. Above all, the component in unfermented plant foods such as spirulina is more likely to be attributed to pseudovitamin B 12 , which has almost no biological effectiveness in mammals.
Vitamin B 12 is contained in almost all foods of animal origin (including eggs and dairy products), at least in small amounts. Compared to cow's milk (0.4 µg / 100 g), breast milk only contains 0.05 µg / 100 g on average. Other sources are vitamin- supplemented foods and nutritional supplements .
According to established expert opinion, no vegetable food for human needs contains sufficient quantities of the usable form of the vitamin. It has been suggested that foods like spirulina , nori , flaky brown algae, and chlorella could provide useful amounts of the vitamin, but the cobalamins they contain are likely not biologically active . All parts of the cobalamin molecule must be present for biological activity. In fact, these foods contain cobalamin analogues, which suppress the cobalamin-dependent enzymes and can cause further deterioration in the B 12 status. Vegetables and legumes preserved by lactic acid fermentation - such as peas , beans and lupins - and zingiberales such as ginger have, albeit low, content of B 12 coenzymes. However, there is currently too little or no evidence that fermented foods can maintain adequate B 12 status.
While searching for potentially suitable sources of vitamin B 12 for vegetarians who also avoid vitamin supplementation, Watanabe et al. on the Korean red alga ( Porphyra sp. ). According to a calculation published by the authors in 2014, the daily consumption of 4 g of the dried seaweed, which in this form is also known under the culinary name nori , could cover a vitamin requirement of 2.4 µg. This statement is based on data from an in vitro digestion simulation of dried Porphyra sp. which were published in 2009. To prove the bioactivity of the cobalamin from nori, an animal experiment with freeze-dried Porphyra yezoensis was published in 2001 . As early as 1991, Dagnelie et al. Tests with nori, spirulina and fermented vegetable food on vitamin B 12 -deficient children and observed a further increase in the mean individual erythrocyte volume . The authors concluded that the bioavailability of the cobalamin from these sources was questionable. They found it unjustified to recommend algae and “other plant foods” as a safe source of vitamin B 12 . The review by Rizzo et al. from 2016 rated the available in-vitro tests as promising, but at the same time pointed out that sufficient human studies are lacking to consider the use of algae in the supply of vitamin B 12 as beneficial.
Manufacturing
For the chemical preparation of vitamin B 12 from Streptomyces cultures or from liver , cyanide is added. The chemical synthesis of vitamin B 12 is very expensive because of the complexity of the cobalamins. Supplements for human consumption or for use in animal feed (poultry and pigs) are therefore mostly produced using genetically modified microorganisms . Since only the cleaned end product comes onto the market, there is no labeling requirement.
requirement
The daily requirement can currently only be estimated. The estimates for adequate intake are much lower than most other vitamins and in the µg ( microgram ) range:
- Infants (up to 12 months): 0.5–1.4 µg / day
- Children (1–15 years): 1.5–4.0 µg / day
- Women: 4.0 µg / day
- Pregnant women: 4.5 µg / day
- Breastfeeding: 5.5 µg / day
- Men 4.0 µg / day
The biological half-life of vitamin B 12 is 450–750 days. The vitamin is constantly released into the small intestine with bile acids and taken up again at its end - the ileum - with the help of the intrinsic factor . The need arises from the quantities that could not be reabsorbed in the ileum, minus the quantities that may already be produced there by microorganisms. If there is a disruption in the formation of the intrinsic factor, the vitamin can only be absorbed or reabsorbed in small quantities, which means that the stores in the organism are quickly emptied. Most cases of vitamin B 12 deficiency are caused by disturbances in the formation of the intrinsic factor.
Healthy adults store 2000–5000 µg of vitamin B 12 in their liver . The filled depot is sufficient to compensate for an undersupply over several years. The situation is different with infants; these have much lower reserves. The liver of a well-fed newborn baby contains only 25–30 µg of the vitamin. The breastfed children of women who follow a vegan diet and do not take sufficient supplements, and whose breast milk is therefore low in vitamin B 12 , usually develop symptoms of deficiency in the second half of their life if they are not fed animal foods or preparations. The high folic acid intake typically associated with a vegetarian diet can mask the haematological symptoms of a vitamin B 12 deficiency so that the deficiency can remain undetected until neurological symptoms appear.
Vitamin B 12 deficiency (hypovitaminosis)
Deficiency symptoms
With a lack of vitamin B 12 may for pernicious anemia ( pernicious anemia ), a disease of the blood count, and the funicular myelosis - damage to the central nervous system with neurological disorders - come. In recent years there has been increasing evidence of a possible connection between a vitamin B 12 deficiency and other clinical pictures such as B. Dementia and Neuropathies. Basically, low vitamin B 12 concentrations in the blood serum are more common in older people.
A deficiency can be caused by insufficient intake with food, by insufficient absorption or by an increased need e.g. B. caused by alcoholism . If the gastrointestinal tract is insufficiently absorbed , the organism lacks the intrinsic factor in gastric juice , a glycoprotein that is produced by the parietal cells of the stomach and is essential for vitamin B 12 absorption. The intrinsic factor binds cobalamin in a complex protected from digestive enzymes and thus enables transport to the intestinal cells, from where vitamin B 12 reaches the outer tissues via binding to other proteins ( transcobalamins ). A disturbance in the uptake in the terminal ileum can also lead to a deficiency.
The first signs of vitamin B 12 deficiency in adults can be tingling and coldness in the hands and feet, exhaustion and weakness, difficulty concentrating and even psychosis .
Typical consequences of a vitamin B 12 deficiency are:
- Methylmalonate aciduria (lack of methylmalonyl-CoA mutase activity)
- Homocystinuria (lack of methionine synthase activity, possibly secondary methionine deficiency)
- megaloblastic anemia (disruption of folic acid metabolism by blocking the N 5 -methyl-THF cleavage to THF)
- Hypersegmented leukocytes (signs of aging due to synthesis problems)
- sensory neuropathy (probably a result of the lack of methylmalonyl-CoA mutase activity and anemia)
Vitamin B 12 , bound to the glycoprotein intrinsic factor derived from the parietal cells of the stomach , is physiologically absorbed in the terminal ileum . After a gastrectomy or in autoimmune gastritis (A- gastritis ), in which the immune reaction to the intrinsic factor-forming parietal cells (= parietal cells ) depends, therefore the uptake of the vitamin B 12 - at least in normal range - hardly possible, so that as a result, a vitamin B 12 deficiency can develop. Even with severe inflammation of the ileum, especially Crohn's disease (= terminal ileum ), but also other intestinal diseases with malabsorption syndrome, or after resection of the terminal ileum or the stomach, there is typically a vitamin B 12 deficiency.
In these cases, regular substitution of vitamin B 12 is necessary, although this should be done by bypassing the gastrointestinal tract in the form of intramuscular, subcutaneous or, rarely, intravenous injection, as either the missing intrinsic factor or the missing or strong one disturbed ileum would largely prevent the absorption of the orally supplied vitamin B 12 . Vitamin B 12 is only absorbed non-specifically when high doses are given . However, the absorption rate cannot be foreseen and therefore oral vitamin B 12 substitution is generally insufficient in these cases.
The first effective preparation for the treatment of pernicious anemia, developed in the 1930s Campolon , based on purified extracts from the livers of animals for slaughter and contained vitamin B 12 as a main component. It replaced the previously practiced liver diet, in which the patients had to eat raw liver or dishes made from it every day.
Test for vitamin B 12 deficiency
Since the turn of the millennium, a CBLA assay ( competitive-binding luminescence assay ) has been carried out to determine the cobalamin level in the blood , which has replaced earlier microbiological and radioisotope dilution assays. This is offered by three manufacturers. Clinical series show that all three assays produce a high rate of false negative results (between 22 and 35%); H. in one out of three to four cases, instead of a cobalamin deficiency, a normal level is incorrectly determined. However, so far only small series are available.
In general, a latent vitamin B 12 deficiency changes u. a. four biomarkers that can be measured in the blood as the deficiency progresses. The most sensitive laboratory value in the case of permanent vitamin B 12 deficiency and the first to become conspicuous is the value for holotranscobalamin (Holo-TC) in the serum. In particular, a Holo-TC of less than 35 pmol / l indicates a vitamin B12 deficiency. If only the holo TC value is reduced, no clinical or haematological symptoms occur at this stage. Establishing a vitamin B 12 deficiency at this stage allows substitution therapy before irreversible neurological damage occurs.
As the deficiency progresses, increased homocysteine levels and increased levels of methylmalonic acid (MMA) can also occur. These values therefore usually only have to be checked if the Holo-TC value is abnormal or if there is any other suspicion of vitamin B 12 deficiency (e.g. with increased creatinine ). The total vitamin B 12 level in the serum is often the most recently declining and therefore relatively unspecific and insensitive bio-marker for the determination of a latent vitamin B 12 deficiency, therefore this parameter for the early determination of a vitamin B 12 - For lack of only limited informative value.
Overdose (hypervitaminosis)
Therapeutic - mostly intravenous - overdoses are associated with local allergic complaints and a form of acne ( Acne medicamentosa ).
Further use
Use as an antidote
Hydroxycobalamin is an antidote for poisoning by cyanide or hydrogen cyanide . Cyanide poisoning occurs mainly in the context of plastic fires. Other causes can be accidental or deliberate ingestion, inhalation or skin contact in industrial accidents.
The clinical symptoms of coma, bradycardia and hypotension of those exposed to smoke in the context of a fire should suggest cyanide intoxication. Like carbon monoxide and nitrous gases , hydrocyanic acid can also be measured in the context of fire fighting and substantiates the suspicion of intoxication. Treatment with 4-dimethylaminophenol (4-DMAP) should be avoided in those exposed to smoke gas, as this, as a Met-hemoglobin-forming agent, has a negative effect on oxygenation.
Under the trade name Cyanokit , Merck KGaA received market approval for hydroxocobalamin from the European Commission on November 29, 2007 via the centralized procedure for the treatment of proven or suspected cyanide poisoning in adults and children. Hydroxocobalamin is a form of vitamin B 12 that binds cyanide ions. This creates cyanocobalamin, which is excreted in the urine via the kidneys. This prevents the cyanide from binding to the cytochrome c oxidase . The starting dose for Cyanokit in adults is 5 g of the active ingredient, which is to be administered as an intravenous infusion. Depending on the severity of the poisoning and the clinical reaction, a second dose of 5 g up to a total dose of 10 g can be administered.
The risk-benefit ratio for cyanide poisoning treatment with hydroxocobalamin is good. A common side effect is a harmless red coloration of the skin and urine that lasts about a week, as well as a slight rise in blood pressure.
Topical application on the skin
Cyanocobalamin alternative medicine for the treatment of atopic eczema (atopic dermatitis) and the psoriasis used (psoriasis). The effect is justified with the results of smaller clinical studies in which an ointment with 0.07% vitamin B 12 (cyanocobalamin) in an avocado oil ointment base was examined.
It is assumed that the effect observed in the studies is due to the fact that cyanocobalamin has the ability to bind nitric oxide, which occurs in increased concentrations in symptomatic skin changes and has a cell-damaging effect.
Before the ointment was launched on the market as a medical product under the brand name Regividerm in November 2009, the television documentary found it undesirable to cure - How pharmaceutical companies prevent a drug , which was broadcast on October 19, 2009 by ARD , a large one and also in the course of it critical media interest. Peter Schönhöfer , a clinical pharmacologist who was commissioned by the WDR to review the studies as part of the production of this documentation, questioned their informative value shortly after the broadcast, as no reliable statement could be made due to the low number of patients. The warnings he had already given to WDR during the production were ignored. The dermatologist and study coauthor Peter Altmeyer expressed to FOCUS , his little side comparison uncontrolled study of 13 psoriasis and 49 patients with atopic dermatitis was hyped by the ARD and been misinterpreted. A field test with a few thousand patients would be necessary to prove its effectiveness.
Since March 2010, the said product may no longer be sold under the name Regividerm , it is now called Mavena B 12 ointment .
See also
literature
- Wolfgang Herrmann, Rima Obeid: Causes and early diagnosis of vitamin B12 deficiency. In: Deutsches Ärzteblatt. Volume 105, No. 40, 2008, pp. 680-685. doi: 10.3238 / arztebl.2008.0680
- Wolfgang Herrmann, Rima Obeid (Ed.): Vitamins in the prevention of human diseases. de Gruyter, 2011, ISBN 978-3-11-021448-2 .
- Wendy PJ den Elzen u. a .: Subnormal vitamin B12 concentrations and anemia in older people: a systematic review. In: BMC Geriatrics. Volume 10, 2010. doi: 10.1186 / 1471-2318-10-42
- aerztezeitung.de: Vitamin B12 may prevent Alzheimer's
- EV Quadros: Advances in the understanding of cobalamin assimilation and metabolism. In: British Journal of Hematology . Volume 148, Number 2, January 2010, pp. 195-204, doi: 10.1111 / j.1365-2141.2009.07937.x . PMID 19832808 . PMC 2809139 (free full text). (Review).
- R. Carmel: Cobalamin, the stomach, and aging. In: The American journal of clinical nutrition. Volume 66, Number 4, October 1997, pp. 750-759. PMID 9322548 . (Review).
Web links
- The absorption of vitamin B12 (overview graphic)
Individual evidence
- ↑ a b c d e f g Estimated values for an adequate intake of vitamin B12 . German Nutrition Society. Retrieved June 1, 2019.
- ↑ a b Data sheet Coenzyme B 12 , ≥97.0% from Sigma-Aldrich , accessed on June 25, 2017 ( PDF ).
- ↑ Entry on cobalamine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Hans Beyer: Textbook of organic chemistry. Leipzig 1968, pp. 617-618.
- ↑ UniProt search result
- ↑ a b c d R. Driesch: Vitamin B12. In: Axel M. Gressner, Torsten Arndt (Hrsg.): Lexicon of medical laboratory diagnostics . 2nd Edition. Springer Verlag, 2012, ISBN 978-3-642-12920-9 , p. 1395. ( limited preview in Google book search)
- ↑ Vitamin B12 In: Pschyrembel Online , Walter de Gruyter GmbH, June 2018.
- ↑ M. Jägerstad & K. Arkbåge: Cobalamins - Properties and Determination . In: Encyclopedia of Food Sciences and Nutrition , 2nd Edition, 2003, pp. 1419-1427. doi : 10.1016 / b0-12-227055-x / 00257-1 .
- ↑ Cyanocobalamin In: PubChem .
- ^ GR Minot, WP Murphy: Treatment of pernicious anemia by a special diet . In: The Yale Journal of Biology and Medicine . tape 74 , no. 5 , 1926, pp. 341-353 , PMC 2588744 (free full text).
- ^ EL Rickes, NG Brink, FR Koniuszy, TR Wood, K. Folkers: Crystalline Vitamin B 12 . In: Science . tape 107 , no. 2781 , April 16, 1948, p. 396 , doi : 10.1126 / science.107.2781.396 , PMID 17783930 (English).
- ↑ K. Folkers: Perspectives from research on vitamins and hormones. In: Journal of Chemical Education. Volume 61, 1984, p. 747. doi : 10.1021 / ed061p747 .
- ^ E. Lester Smith: Purification of Anti-pernicious Anæmia Factors from Liver . In: Nature . tape 161 , no. 4095 , April 1948, p. 638 , doi : 10.1038 / 161638a0 , PMID 18856623 (English).
- ↑ a b H. Martens, M. Barg, D. Warren, J.-H. Jah (2002): Microbial production of vitamin B 12. In: Applied Microbiology and Biotechnology , 58 (3), pp. 275-285. doi : 10.1007 / s00253-001-0902-7 . PMID 11935176 .
- ↑ Manuscript of your lecture on the occasion of the award of the Nobel Prize (PDF; 343 kB).
- ↑ Walter Schilling (1974) Total synthesis of vitamin B-12: Representation of intermediate products and partially synthetic final stages , doctoral thesis (from the laboratory of Eschenmoser / ETH), accessed on Aug. 25, 2019
- ^ Hans-Dieter Belitz , Werner Grosch , Peter Schieberle : Textbook of food chemistry . 6th completely revised edition. Springer, Berlin / Heidelberg 2008, ISBN 978-3-540-73201-3 , p. 427 , doi : 10.1007 / 978-3-540-73202-0 .
- ^ I. Ahmad, K. Qadeer, S. Zahid, MA Sheraz, T. Ismail, W. Hussain, IA Ansari: Effect of ascorbic acid on the degradation of cyanocobalamin and hydroxocobalamin in aqueous solution: a kinetic study. In: AAPS PharmSciTech . Volume 15, number 5, October 2014, pp. 1324-1333, doi: 10.1208 / s12249-014-0160-5 , PMID 24920523 , PMC 4179674 (free full text).
- ↑ Methionine Synthase (diagram).
- ↑ a b Mechthild Busch-Stockfisch: Food Lexicon. 4th edition. Behr's Verlag DE, 2005, ISBN 3-89947-165-2 .
- ↑ H.-D. Belitz , W. Grosch, P. Schieberle: Textbook of food chemistry. 6th edition. Springer, 2007, ISBN 978-3-540-73201-3 , p. 418.
- ↑ Entry on vitamin B 12 . In: Römpp Online . Georg Thieme Verlag, accessed on February 11, 2013.
- ↑ a b c Claus Leitzmann u. a .: 21. Vitamin B 12 (cobalamin). In: Nutrition in Prevention and Therapy: A Textbook. 2nd Edition. Georg Thieme Verlag, 2003, ISBN 3-8304-5273-X , p. 51. ( limited preview in Google book search)
- ↑ a b The information does not correspond to the content of the vitamin in the food that can be used by the human organism. Above all, the component in unfermented plant foods such as spirulina is more likely to be attributed to pseudovitamin B 12 , which has almost no biological effectiveness in mammals
- ↑ a b Geoffrey P. Webb: Dietary supplements and functional foods. Wiley-Blackwell, 2006, ISBN 1-4051-1909-8 , p. 196.
- ↑ Anatol Schmidt, Lisa Call, Lukas Macheiner, Helmut K. Mayer: Determination of vitamin B12 in four edible insect species by immunoaffinity and ultra-high performance liquid chromatography . In: Food Chemistry . 2018. doi : 10.1016 / j.foodchem.2018.12.039 .
- ^ Gerhard Habermehl, Peter E. Hammann, Hans C. Krebs: Naturstoffchemie. An introduction. 2nd Edition. Springer, Berlin 2002, ISBN 3-540-43952-8 .
- ↑ Zenon Schneider: Comprehensive B12. Walter de Gruyter, 2011, ISBN 978-3-11-084479-5 , p. 189.
- ↑ a b Wolfgang Löscher, Fritz Rupert Ungemach, Reinhard Kroker: Vitamin B12. In: Pharmacotherapy in pets and farm animals. 7th edition. Georg Thieme Verlag, 2006, ISBN 3-8304-4160-6 , p. 346. ( limited preview in Google book search)
- ↑ H. Lindermayer, G. Probstmeier, W. Preißinger: Principles of pig feeding , teaching and advisory aid . Part 1: Basics of nutritional physiology. (PDF) LfL Animal Nutrition, Bavarian State Institute for Animal Nutrition, 2009, p. 44.
- ↑ A. Domke et al. a. (Ed.): Toxicological and nutritional aspects Part I - Use of vitamins in food. Federal Institute for Risk Assessment , Berlin 2004, ISBN 3-931675-87-4 , p. 212, full text (PDF; 1.3 MB).
- ↑ H.-P. Klöcking: 8.3.2 Vitamin Deficiency Anaemia. In: Hans-Hasso Frey, Wolfgang Löscher (Hrsg.): Textbook of pharmacology and toxicology for veterinary medicine. 2nd Edition. Georg Thieme Verlag, 2007, ISBN 978-3-8304-1070-6 , p. 216. ( limited preview in Google book search)
- ↑ J. Köhrle, L. Schomburg: 11.4 Kobalt. In: Hans-Konrad Biesalski, Stephan C. Bischoff, Christoph Puchstein (eds.): Nutritional medicine: according to the new nutritional medicine curriculum of the German Medical Association. 4th edition. Georg Thieme Verlag, 2010, ISBN 978-3-13-100294-5 , p. 205. ( limited preview in Google book search)
- ↑ Entry on human milk. In: Römpp Online . Georg Thieme Verlag, accessed on May 1, 2013.
- ↑ a b c d Lindsay H. Allen: Causes of vitamin B 12 and folate deficiency. In: Food and Nutrition Bulletin. vol. 29, no. 2 (supplement), The United Nations University, 2008, p. S21. Full text (PDF)
- ↑ a b W.J. Craig, AR Mangels; American Dietetic Association: Position of the American Dietetic Association: vegetarian diets. In: Journal of the American Dietetic Association. Volume 109, Number 7, July 2009, pp. 1266-1282. PMID 19562864 , full text (PDF; 644 kB)
- ↑ Fumio Watanabe, Yukinori Yabuta, Tomohiro Bito, Fei Teng: Vitamin B 12 -containing plant food sources for vegetarians. In: Nutrients. 6 (5), May 2014, pp. 1861–1873. doi: 10.3390 / nu6051861 PMID 24803097
- ↑ PC Dagnelie, WA van Staveren, H. van den Berg: Vitamin B-12 from algae appears not to be bioavailable. In: American Journal of Clinical Nutrition. 53 (4), Apr 1991, p. 988. PMID 2000824 .
- ↑ Gianluca Rizzo et al .: Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. In: Nutrients. 8 (12), Dec 2016, p. 767. doi: 10.3390 / nu8120767 . PMID 27916823 . PMC 5188422 (free full text).
- ↑ Eckhart Buddecke: 8. Cobalamin In: Outline of Biochemistry: For students of medicine, dentistry and natural sciences , Walter de Gruyter GmbH & Co KG, Reprint 2018; P. 363. ISBN 978-3-11-163720-4 .
- ↑ transgen.de Website of the Forum Bio- und Gentechnologie e. V. on vitamin B12 (cobalamin)
- ↑ Questions and answers from the DGE on vitamin B12 . German Nutrition Society. Retrieved June 1, 2019.
- ↑ a b Berthold Koletzko, Franziska Feldl: Balanced substrate supply through meat consumption. In: Dtsch Arztebl. 95 (11), 1998, pp. A-606 / B-494 / C-466. (Full text)
- ^ Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline: 9. Vitamin B12. In: Dietary Reference Intakes for Thiamine, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press, Washington DC 1998, ISBN 0-309-06411-2 , p. 324. (online)
- ↑ Ralph Carmel, Yash Pal Agrawal: Failures of Cobalamin Assays in Pernicious Anemia . In: New England Journal of Medicine . 367, 2012, pp. 385-386.
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , pp. 31–33.
- ↑ a b Wolfgang Herrmann, Rima Obeid: Causes and Early Diagnosis of Vitamin B12 Deficiency. In: Deutsches Ärzteblatt . Volume 105, Issue 40, 2008, pp. 680-685. doi: 10.3238 / arztebl.2008.0680
- ↑ F. vd Berg, L. Gifford, J. Cabri, L. Arendt-Nielsen, E. Bader: Applied Physiology: Understanding and influencing organ systems. 2nd Edition. Thieme, 2005, ISBN 3-13-117082-4 , p. 236.
- ^ O. Braun-Falco , H. Lincke: The problem of vitamin B6 / B12 acne. A contribution on acne medicamentosa. In: Munich medical weekly. Volume 118, 1976, pp. 155-160. PMID 130553
- ↑ Cyanokit (R) approved by Merck Serono in the European Union. Press release from November 29, 2007.
- ↑ Wolfgang Uhl, Arno Nolting, Georg Golor, Karl Ludwig Rost, Andreas Kovar: Safety of Hydroxocobalamin in Healthy Volunteers in a Randomized, Placebo-Controlled Study. In: Clinical Toxicology. 44, 2006, pp. 17-28. doi: 10.1080 / 15563650600811755
- ↑ M. Stücker et al. a .: Vitamin B12 Cream Containing Avocado Oil in the Therapy of Plaque Psoriasis. In: Dermatology. Volume 203, 2001, pp. 141-147. PMID 11586013 ; doi: 10.1159 / 000051729 .
- ↑ M. Stücker et al. a .: Topical vitamin B12 - a new therapeutic approach in atopic dermatitis-evaluation of efficacy and tolerability in a randomized placebo-controlled multicentre clinical trial. In: British Journal of Dermatology . Volume 150, No. 5, 2004, pp. 977-983. PMID 15149512 ; doi: 10.1111 / j.1365-2133.2004.05866.x
- ↑ R. Januchowski: Evaluation of topical vitamin B (12) for the treatment of childhood eczema. In: Journal of alternative and complementary medicine. Volume 15, Number 4, April 2009, pp. 387-389, doi: 10.1089 / acm.2008.0497 . PMID 19368512 .
- ↑ Avoxa Media Group for German Pharmacists: Vitamin B12 Cream: More than "pink chicken poop"? In: Pharmaceutical newspaper online. 2009, accessed March 4, 2017 .
- ↑ Winfried Köppelle: Surreptitious advertising for quack salve and wonder book. In: Laborjournal. November 2009, pp. 68-70. (Full text version) ( Memento of the original from November 15, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b Jochen Niehaus: Applied too thickly. In: Focus Online. October 26, 2009; Retrieved November 16, 2009.
- ↑ Psoriasis Therapy - Pink is hope. In: Frankfurter Allgemeine Zeitung.
- ^ Atopic dermatitis cream - false promises of healing. Eco test
- ↑ NDR , ZAPP magazine from October 28, 2009.
- ↑ Name change of our vitamin B12 ointment. ( Memento from February 18, 2012 in the Internet Archive ) Website of Regeneration Pharma GmbH, as of February 18, 2012.