High speed steel

High-speed steel is a high-alloy tool steel that is mainly used as a cutting material , i.e. for milling tools , drills , turning tools and broaching tools . The designation refers to the cutting speeds three to four times higher than normal tool steel . While ordinary tool steel loses its hardness from around 200 ° C, high-speed steel retains its hardness up to around 600 ° C. The common abbreviations begin with HSS or HS , derived from the English High Speed Steel . German terms are high-speed steel , (high-performance) high-speed steel , high- speed high-speed steel and high-performance cutting steel .
The terms AHSS and HSS are not directly related, although both are tool steels . AHSS stands for Advanced High Strength Steel ( German about: More advanced high-strength steel ) and refers to modern high-strength unalloyed cold work steels .
After its development in 1906, high-speed steel very quickly replaced conventional tool steel ( cold-work steel ) in cutting tools and has now almost completely replaced it.
Of all cutting materials used in modern, industrial machining technology such as u. a. Carbide , cermets or boron nitride , high-speed steel has the highest breaking strength and the best grindability , but the lowest hot hardness and wear resistance , so that even higher cutting speeds are possible with other cutting materials.
The most important alloying elements are carbon , tungsten , molybdenum , vanadium , chromium and cobalt , the proportion of which can amount to over 30%. Like all other tool steels, high-speed steel gets its hardness from its martensitic basic structure, which consists of iron and carbon. The other alloy elements ensure better wear resistance and the resistance of the martensite up to temperatures of 600 ° C. The high hot hardness and high heat resistance compared to ordinary tool steel is based on the heat treatment: First, the high-speed steel is annealed at over 1200 ° C and then quenched in order to create the martensitic basic structure. This is followed by repeated tempering at around 550 ° C, during which the brittleness is reduced and tiny carbides (tungsten / molybdenum / vanadium-carbon compounds) precipitate from the martensite , which are responsible for the hot hardness and heat resistance.
Applications, comparison with common tool steel and other cutting materials
Various cutting tools are made from high-speed steel . Among them are turning tools , milling cutters , drills and reaming tools . The last two are often made of high-speed steel; Other cutting materials are more widely used for turning tools and milling tools, especially hard metal , which of all cutting materials is most similar to high-speed steel. HSS tools are preferred for more complex tool shapes such as profile tools because they can be ground very well. Ordinary tool steel (cold work steel) is only used for tools that cannot achieve high cutting speeds, such as files and rasps , as well as for tools for woodworking . Otherwise, cold work steel has been completely replaced by high speed steel in machining. Advantages of HSS tools compared to carbide tools are the lower price, the good grindability, which enables complicated tool shapes and also allows the regrinding of blunt tools, as well as the higher strength: HSS tools are less sensitive to breakage when subjected to shock loads.
Cold work steel loses its hardness at temperatures from 200 ° C and is therefore unusable as a cutting material. The effect limits the cutting speed when machining steel to around 5 m / min. High-speed steel retains its hardness up to around 600 ° C, which allows cutting speeds of up to around 80 m / min. Tools made of hard metal, cutting ceramics and boron nitride are suitable for temperatures of over 1000 ° C. In industrial practice, the cutting speed is no longer limited by temperature, but by wear. HSS tools can be coated with titanium nitride and other substances that increase wear resistance, which above all increases the service life of the tools and allows slightly higher cutting speeds.
history
After the need for a cutting material that enables higher cutting speeds than tool steel increased at the end of the 19th century, Taylor and White developed the high-speed steel by 1906, which quickly became established. From the 1930s and increasingly since the 1960s, it was replaced by carbide and other cutting materials, but was never completely replaced. The exact metallurgical relationships to explain the high hot hardness of high-speed steel could only be clarified in the middle of the 20th century.
initial situation
In the course of the industrial revolution , workpieces made of steel were increasingly machined, whereas previously wood and softer metals such as copper and tin were mainly machined. Steel is much stronger than these materials and led to much higher wear of the tools, which consisted of ordinary tool steel, so that the economically sensible cutting speeds were only 5 m / min. Tool steel had long been known, consisted only of iron and carbon and could already be hardened at 750 ° C to 835 ° C, but lost its hardness at around 200 ° C. The first improvement was made in 1868 by Robert Mushet with a tool steel alloyed with 6 to 10% tungsten, 1.2 to 2% manganese and 0.5% chromium . For hardening, it could be cooled in air instead of in a water bath, which was known as self-hardening. When hardening in water, the workpieces repeatedly broke. With Mushets tool steel, cutting speeds of up to 7 m / min could be achieved.
In the 1860s, with the development of the Siemens-Martin process and the Bessemer process, it became possible to generate temperatures of over 1800 ° C. This made it possible for the first time in history to melt steel, which improved its degree of purity. Metals that melt at even higher temperatures, such as tungsten (over 3300 ° C), could be produced with the then new electrochemistry , so that they were available as alloying elements . At economic cost they were only available at the turn of the century after the expansion of the electrical power supply.
Developed by Taylor and White
At the turn of the century, the American engineer Frederick Winslow Taylor began looking for ways to make production more economical. From this he developed, among other things, organizational principles known as Taylorism or Scientific Management . He also researched machining technology for about 10 years and in 1906 wrote his book On the Art of Cutting Metals (literally: About the art / technology of cutting metal ), in which he explained for the first time general relationships in machining technology, including the Taylor named after him - Straight line that indicates a relationship between the tool life and the cutting speed, as well as relationships between the tool geometry , the feed rate, the cutting depth and cooling lubricants. Together with the metallurgist Maunsel White, he determined the cutting speeds for various tool materials that led to a tool life of 20 minutes in standardized tests. After that, they turned to heat treatment and tool assembly. For different chemical compositions, they varied the level of the annealing temperature, which could reach the melting temperature, as well as the level of the temperatures at which they were then further treated by tempering and then determined the cutting speeds for the 20-minute service life. In 1906 the best known composition was 0.67% C, 18.91% W, 5.47% Cr, 0.11% Mn, 0.29% V and the remainder of iron. The most favorable heat treatment was for annealing at 1250– 1290 ° C, just below the solidus temperature at which the material begins to melt, followed by quenching in liquid lead (620 ° C) and then further cooling to room temperature. Then they were tempered at just under 600 ° C. The tools retained their hardness up to around 600 ° C and enabled cutting speeds of up to 30 m / min when machining steel. Taylor introduced this high-speed steel to the public at the 1906 World's Fair .
exploration
Taylor and White knew they had discovered an entirely new mechanism for hardening steel, but it wasn't until the 1950s that the exact function was clarified through the use of electron microscopes . When tempering, tiny particles between 400 ° C and 600 ° C separate from the basic structure, which have a diameter of only 0.05 µm and are therefore not visible under normal optical microscopes. High-speed steels were thus the first materials to be treated by precipitation hardening , and were about 10 years ahead of the aluminum alloys that are known today for this hardening mechanism.
distribution
After the World's Fair of 1906, Taylor was a world famous man. There he demonstrated the turning of steel at cutting speeds that were so high that the cutting edges of his tools began to glow red and yet did not fail. It is estimated that in the US alone, HSS tools were sold for a total of $ 20 million in the next few years, which, thanks to the higher cutting speeds, led to increases in production worth $ 8 billion. However, the machine tools of that time were not designed for the high loads. In tests by the then famous German company Ludwig Loewe , the machines were completely unusable after a few weeks. In Europe, high-speed steel was initially used at normal cutting speeds, where it led to significantly longer tool lives than when using tool steel. The machine manufacturers soon offered machines that were designed for the higher cutting speeds; However, since the old ones were very durable when used properly, the changeover was initially slow. Only when a new generation was offered in the 1920s that also had electrical drives and controls instead of the previously common mechanical controls and drives with steam engines, the high possible cutting speeds were exhausted.
In the 1930s, carbide was developed as a new cutting material with which cutting speeds about three times higher than with HSS were possible. Use was initially slow for cost reasons and because the machines were again not designed for the even higher loads. From the 1960s, carbide became the industrial standard, but it could not completely replace high-speed steel, nor could the cutting ceramics , boron nitride and diamond tools developed later . High speed steel continued to be improved by the 21st century, but the increases in cutting speed are small. Significant improvements are not expected.
Structure and compositions
The fabric , so the microstructure is crucial for the mechanical properties of high speed steel, as with most materials. The structure depends on the type and amount of alloying elements and the state of heat treatment.
Structure in the state of use
After casting and during machining of the HSS tools, the structure is similar to that of normal steels; it also contains particles of alloy elements with sizes of a few micrometers, but these have no particular influence. In the case of finished HSS workpieces, the material is in a quenched and tempered state. As with all tool steels , the basic mass consists of martensite , which mainly consists of iron and carbon. The small carbon atoms are located in the gaps between the much larger iron atoms, so that they can hardly move, which is why martensite is very hard and wear-resistant. In addition, parts of the remaining alloying elements are bound in the martensite, which leads to the high strength of high-speed steel through solid solution strengthening . All of the iron is in martensite, but only part of the carbon; the remaining part combines with the alloying elements (except cobalt) to form carbides , compounds that are significantly harder than martensite. Their hardness does not play a major role in the overall hardness of high-speed steel. Carbide-free martensite transforms into softer forms of steel from around 200 ° C. The carbides themselves are thermally very stable and only dissolve again in their basic structure at over 1000 ° C. The carbides are in the form of tiny particles (grains). The larger ones with diameters in the micrometer range are created after casting, they make up about 10 to 15% of the volume. Some of them still exist at temperatures above 1290 ° C and ensure that high-speed steel can be annealed at these temperatures without reducing its strength due to grain growth . The smaller particles are significantly smaller than a micrometer (approx. 0.05 µm), are very numerous, arise as precipitation during tempering, are located near the grain boundaries of the martensite grains and prevent diffusion , which would start at higher temperatures and cause a drop Would cause hardness and strength. They also tension the martensite lattice so that it can only transform at temperatures above 600 ° C.
Alloy elements

In addition to the carbon found in all steels, high-speed steel contains a number of other alloying elements. Tungsten and molybdenum, which are interchangeable with one another, are of the greatest importance. Except for cobalt, they all combine with carbon to form carbides. Molybdenum and tungsten together with iron form the double carbide Fe 3 (W, Mo) 3 C with a Vickers hardness of 1,150 HV.
The alloy elements have the following effects and tasks:
- Tungsten : Forms carbides (especially tungsten carbide ), increases hot hardness, tempering resistance and wear resistance.
- Molybdenum : Can replace tungsten and has the same effect as tungsten at half its mass. Molybdenum forms carbides, improves through hardening, toughness, hot hardness, tempering resistance and wear resistance. Molybdenum-rich types have to be subjected to a more complex heat treatment, which is, however, state of the art. They are also usually cheaper.
- Vanadium : Forms vanadium carbide , which, like in some other steels, increases the wear resistance due to its hardness (2000 HV).
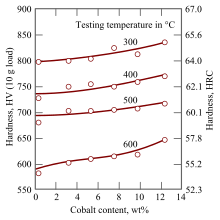
- Cobalt : Increases the temperature up to which the material can be used, i.e. from which the martensite is transformed. It hinders the growth of the excreted carbides.
- Chromium : Participates in the formation of carbides and improves hardenability. This means that tools with larger cross-sections can also be hardened.
- Carbon : It is needed on the one hand to form martensite and on the other hand to form the carbides. Its proportion is adjusted to the proportions of the other elements.
Varieties and names
The designations begin with "HS" (DIN EN ISO 4957) or "S" followed by a sequence of digits that indicate the percentage of the metallic alloy elements in the order tungsten-molybdenum-vanadium-cobalt (W-Mo-V-Co). The standard type HS6-5-2, for example, contains 6% tungsten, 5% molybdenum, 2% vanadium (no cobalt), chromium according to the standard, a suitable amount of carbon and otherwise iron. In English-speaking countries, they are divided into two groups: Tungsten-rich types begin with a T (from English Tungsten = tungsten), molybdenum-rich types begin with an M. In both cases, a number follows to differentiate between different types, but this has no further meaning. Powder metallurgically produced grades are given the addition PM. They have higher strengths, but are more expensive.
The high-speed steels are divided into a total of four groups, which differ in the amount of tungsten and molybdenum.
- The first group contains 18% tungsten and almost no molybdenum. These include the types HS18-0-1 and 18-1-2-5.
- The second group contains 12% tungsten and up to 4% molybdenum. Examples are the types HS 12-1-4-5 and HS-10-4-3-10.
- The third group contains 6% tungsten and 5% molybdenum. This includes the standard grade HS6-5-2, as well as HS6-5-3 or HS6-5-2-5.
- The fourth group contains a maximum of 2% tungsten and 9% molybdenum. It includes the types HS2-9-1, HS2-9-2 or HS2-10-1-8.
The molybdenum-rich varieties are particularly tough and insensitive to sudden loads.
The vanadium content varies between 0 and 4%, the cobalt content is either zero or at least 5% for tools with increased temperature requirements. All grades contain 4-5% chromium.
properties
Hardness and hot hardness
The hardness at room temperature is similar to normal hardened steel with values of 800 to 900 HV ( Vickers hardness ), or about 65 HRC (Rockwell). It decreases only slightly up to around 400 ° C, then faster; from 600 ° C, the hardness drops rapidly to values that are no longer usable for tools. Ordinary tool steel loses its hardness at around 200 ° C. The competing hard metals reach hardnesses of 1300 to 1700 HV, cutting ceramics even 1400 to 2400. Both keep them up to temperatures of 1000 ° C and higher.
Strength, flexural strength and heat resistance
The compressive strength at room temperature is about 2000 to 3000 N / mm 2 , at 600 ° C it is still 1700 N / mm 2 . Tool steels are somewhat lower, hard metals higher with values of 4000 to 5900 N / mm 2 at room temperature.
The flexural strength at room temperature for high-speed steel is around 2500 to 3800 N / mm 2 , which is the highest value of all cutting materials. The flexural strength primarily determines the sensitivity to breakage under sudden loads. Hard metals reach values of 800 to 2200 N / mm 2 , cutting ceramics only 300 to 700 N / mm 2 .
Wear resistance and wear
Various wear mechanisms occur in machining technology. Depending on the temperature at the cutting edge, they are differently pronounced.
Plastic deformation
At high temperatures (over 600 ° C) and high compressive or shear forces, plastic (permanent, non-elastic) deformations occur. The forces and temperatures required for this only occur when machining high-strength materials such as high-alloy steel, titanium or nickel , and especially with their alloys. High temperatures occur especially at high cutting speeds. This type of wear is seldom seen in industrial practice because the tools wear out extremely quickly; However, this type of wear represents the limiting factor for the cutting speed. Deformation can occur both as crater wear (on the rake face ) and as a deformation of the cutting edge. The cause is also the hardening effect at high forming speeds as a result of the high cutting speeds, which lead to higher forces and thus also temperatures.
diffusion
Diffusion is a wear mechanism that only occurs at high temperatures and is also rare in industrial practice. The alloying elements of the tool migrate into the chip, while undesired elements migrate from the chip into the tool. Both effects reduce the strength of the tool. Diffusion wear occurs in high-speed steel at high temperatures, but the wear due to plastic deformation is much greater.
Bonding (adhesion)
At low cutting speeds, the chip may stick ( adhesion ) to the tool. When the following workpiece material hits the bond, parts of the tool surface can also be torn out by micro-welds. In addition, built- up edges can form at low cutting speeds , which also has negative effects. Bonding occurs frequently with high-speed steel.
Abrasion
Abrasion ( Abrasive wear ) is the main cause for the wear of high-speed tools. If the material of the workpiece contains particles that are harder than the martensite of the tool, then these particles act like abrasive grains and separate material from the tool. Many workpiece materials contain such particles, in steel, for example, oxides , nitrides and carbides . At castings often sand grains adhere to. Certain carbides are also torn out of the tool by sticking and carried over the tool surface together with the chip.
Machinability
In the unhardened state, high-speed steel can be machined well (see Machinability of steel ), at high temperatures it can also be forged, but it is significantly heavier than most other types of steel. In the hardened state, high-speed steel can practically only be machined by grinding ; however, it is very easy to grind, while competing cutting materials are very difficult to grind. Ordinary grinding wheels are sufficient for HSS, while diamond grinding wheels are required for hard metals and cutting ceramics.
Manufacturing and heat treatment
There are two different methods of manufacturing high-speed steels: the melt metallurgical method , which accounts for the majority of the production volume, and the powder metallurgical method . In the first variant, the raw material is melted and cast into bars; in the second, powder is used as the starting material. In both variants, the material is then forged and shaped by milling and drilling. A variant of powder metallurgical production is "die pressing", in which the material is pressed directly into molds without forging and machining. All variants end with heat treatment and the subsequent grinding of the final contour.
Melt metallurgical production
The steel is first melted at 1550 ° C and the alloying elements are added. The melt is then poured into ingot form. Since low-melting components solidify first and are therefore unevenly distributed (so-called segregation , particularly pronounced in high-alloy materials such as high-speed steel), block annealing is then carried out at 900 ° C in order to distribute the components more evenly (homogenize). In addition, electroslag remelting (ESR) can take place, in which only a small part of the block is melted and this liquid point is passed through the block. The material gains in purity and becomes more uniform, but the process step is relatively expensive. This is followed by rolling and forging, which is used to crush the various hard materials ( ledeburite and carbides) and takes place at temperatures around 1200 ° C. This is followed by soft annealing to enable the subsequent forging steps and machining (milling, drilling), in which the rough shape of the tools is worked out.
Powder metallurgical production
There are two ways to produce the powder: water spraying and gas spraying.
- Gas-atomized powder is filled into capsules and hot isostatically pressed , i.e. at high temperatures and at constant pressure. This is followed by hot forming (rolling, forging) and machining, followed by heat treatment.
- There are two further processing methods for water-atomized powder:
- Cold isostatic pressing at room temperature. The porosity is not completely eliminated. This is followed by sintering , a kind of annealing just below the melting temperature, at normal ambient pressure. This is followed by rolling and forging to remove the pores, then machining and finally heat treatment.
- Die presses : This variant is used for the manufacture of indexable inserts . The powder is pressed into molds and completely compacted, so the porosity is completely eliminated. The panels are then reworked by hot isostatic recompression and finally heat treated. There is no rolling, forging or machining here. This variant is therefore similar to the manufacture of the indexable inserts from hard metal .
Heat treatment
The heat treatment is the crucial step in which the high speed steel receives its hot hardness and strength. Also, it's the final step before finishing by sanding. The heat treatment consists of two sub-steps: annealing and quenching , as well as the subsequent tempering . These processes are also used with many other steels, but there are some special features with high-speed steel.
The aim of annealing is to dissolve as many alloying elements as possible in the ferrous basic structure. The solubility generally increases with the temperature, but with steel it is crucial that the microstructure changes at high temperatures (depending on the carbon content) and is in the form of austenite , in which the solubility is significantly greater. The workpieces are slowly heated to the annealing temperature, as the thermal conductivity of high-speed steel is low and the heat inside the workpieces only increases slowly. In addition, if the outside and inside temperatures differ greatly, there would be thermal stresses that would destroy the workpieces through cracks. The higher the temperature, the more alloying elements can be dissolved and the longer the annealing, the greater the proportion that is actually dissolved. However, as the temperature increases and the duration of annealing increases, the undesirable effect of grain growth , which reduces strength, is accelerated. Annealing takes place at about 1200 ° C. Even at these high temperatures, some of the alloying elements are still present in the form of carbides, which limit the grain growth, which is why high-speed steel is one of the few steels that can be annealed at these temperatures without significant loss of strength (i.e. without grain growth).
After the required annealing time, the material is cooled down relatively quickly (quenching). This creates the martensitic basic structure that gives high-speed steel its hardness. Because of the high cooling rates, more alloying elements remain dissolved than can actually be dissolved at room temperature. The iron atoms can therefore hardly move. An important side effect for the subsequent tempering is that the alloying elements are finely distributed over the entire material and are not accumulated in particles, as is the case with slow cooling. A small part of the structure is still in the form of austenite (retained austenite).
During the subsequent tempering , different processes take place in the individual structural components, which depend on the level of the temperature. Up to about 400 ° C only the martensitic basic structure changes. The forcibly released elements leave the iron lattice and the hardness decreases, only insignificantly at low temperatures and faster and faster at higher temperatures. Above all, carbon is precipitated from the martensite up to around 350 ° C, above this also the metallic alloying elements, which combine with the carbon to form the carbides. Since the alloying elements are finely divided, they form numerous tiny particles and thus lead to precipitation hardening . From around 450 ° C, finely divided carbides also separate from the retained austenite. Because of the lower proportion of alloying elements in retained austenite, it is now converted into martensite (secondary artensite), which also leads to an increase in hardness. The increase in hardness due to the precipitation of carbides and the formation of secondary artensite is greater than the loss of hardness of the original martensite. Tempering is usually carried out at temperatures around 560 ° C and repeated once or twice.
Surface treatments and coatings
The surfaces of the HSS tools can either be subjected to a surface treatment or they are coated. Both of these increase the wear resistance and thus the service life and, to a small extent, the applicable cutting speed. The following procedures are used:
- Nitriding increases the nitrogen content on the surface.
- Steam tempering forms a thin layer of iron (II, III) oxide .
- Chrome plating , application of a layer of chrome approximately 50 to 70 µm thick.
- PVD coating (physical vapor deposition), with titanium nitride or titanium aluminum nitride .
- Which is hardly used CVD processes (chemical vapor deposition), because the needed temperatures well above 600 ° C. It is only used for indexable inserts made of HSS, which hardly warp. After coating, they have to be post-cured.
The coatings also increased the wear resistance when they were partially removed by regrinding of previously worn tools.
Individual evidence
- ↑ What is tool steel? , In: Kontur-Werkzeugstahl.de
- ^ Alfred Herbert Fritz, Günter Schulze: Manufacturing technology. Springer, 11th edition, 2015, p. 296 f.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, drilling, milling. 8th edition, Springer 2008, pp. 110-113.
- ^ Trent, Wright: Metal Cutting. Butterworth Heinemann, 2000, 4th edition, p. 132.
- ^ Trent, Wright: Metal Cutting. Butterworth Heinemann, 2000, 4th edition, pp. 133, 138.
- ^ Trent, Wright: Metal Cutting. Butterworth Heinemann, 2000, 4th edition, p. 139.
- ^ Trent, Wright: Metal Cutting. Butterworth Heinemann, 2000, 4th edition, p. 138.
- ^ Trent, Wright: Metal Cutting. Butterworth Heinemann, 2000, 4th edition, p. 142 f.
- ^ Trent, Wright: Metal Cutting. Butterworth Heinemann, 2000, 4th edition, pp. 139 f.
- ^ Fritz, Schulze: Manufacturing Technology , 9th Edition, p. 275.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 140.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 142.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 117 f.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 142. "the particles remain stable for many hours and harden the steel by blocking the dislocations which facilitate slip between the layers of iron atoms"
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 111 f.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 110.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 140.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 111 f.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 143.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 143.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 143.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 141.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 110 f.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 143.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 110 f.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 144.
- ^ Fritz, Schulze: Manufacturing Technology , 11th Edition, p. 296.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, p. 145.
- ^ Fritz, Schulze: Manufacturing Technology , 11th Edition, p. 296.
- ^ Fritz, Schulze: Manufacturing Technology , 11th Edition, p. 296.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, pp. 150-155.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, pp. 155-157.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, pp. 157-159.
- ^ Trent, Wright: Metal Cutting . Butterworth Heinemann, 2000, 4th edition, pp. 159-161.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 113.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 114.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, pp. 113–115.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, pp. 115-118.
- ↑ Denkena: Spanen , 3rd edition, p. 174.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, milling, drilling. 8th edition. Springer, Berlin 2008, p. 118.
- ↑ Denkena: Spanen , 3rd edition, p. 174.
- ↑ Denkena: Spanen , 3rd edition, p. 174.