Martensite
Martensite is a metastable structure in metals and also non-metals, which is created without diffusion and athermally through a cooperative shear movement from the initial structure. The material has to be cooled (mostly quenched) from the temperature of a high-temperature phase (for steel: γ-phase, austenite ) below the equilibrium temperature to a low-temperature phase (for steel: α-phase, ferrite ). The subcooling below the equilibrium temperature must be deep enough to generate the necessary driving force for the athermal phase transition (see Figure 3), but must also take place quickly enough to prevent diffusion processes (see time-temperature conversion diagram ). The necessary subcooling and cooling rate are strongly dependent on the material under consideration (in the case of steel: on the alloying elements ) and vary over a wide range, so that sometimes rapid quenching in water and possibly subsequent deep cooling in liquid nitrogen (because of the Leidenfrost effect is a direct quenching with liquid nitrogen is not possible), sometimes slow cooling in air or in a hot bath is sufficient.
If the high-temperature phase is metastable conserved at room temperature, it can be transformed into martensite due to stress or strain (see retained austenite transformation in steels). Reversible martensitic transformations as a basic phenomenon of the shape-memory effect also belong to this category.
Martensitic transformations occur in unalloyed and alloyed steels as well as in many non-ferrous metals, ceramics and polymers and are not a phenomenon that is restricted to metals. For steels, martensitic transformation is a frequently used option for influencing properties (see hardening and tempering ).
The structure is named after the German metallurgist Adolf Martens (1850–1914).
Martensite in the iron-carbon system
Martensite is created in steel by a diffusion-free flipping process from the face-centered cubic lattice of austenite into a tetragonal, body-centered lattice , during rapid cooling to a temperature below the martensite start temperature M S (martensite start) . The conversion stops when the cooling is stopped. If the martensite finish temperature M F (martensite finish) is reached, the volume fraction of the martensite does not increase any further with further cooling.
This folding process or this cooperative shear movement means that the martensite lattice is only created from the initial lattice through orderly changes in angle and position. The individual atoms move only a fraction of the distance between them. The central rib of each martensite plate that is created, the so-called invariant habitus plane , does not take part in the folding down (see Figure 8).
Depending on the amount of stored carbon, some of the austenite is always not converted. This retained austenite can be explained by the high distortion stresses that the martensite plates created last exert on the martensite plates created before them and thereby prevent them from further growth. The martensite plates have a lenticular or needle-shaped cross-section and run through from one side of the grain to the other at the start of martensite formation, see Figure 1. Further plates then grow at different angles, but mostly perpendicular to those already in the grain.
The carbon dissolved in the austenite remains forcibly dissolved in the mixed crystal due to the rapid cooling during quenching . As a result, the folded car grille is distorted tetragonally , creating a very hard structure. The cooling rate at which the first fractions of martensite (in addition to ferrite , pearlite , bainite ) arise is called the lower critical cooling rate. If only martensite is formed for the first time during cooling, the upper critical cooling rate has been reached.
Martensite is used in steels to achieve a significant increase in hardness. The higher the carbon content of the martensite, the higher the hardness (up to approx. 0.6% C; however, the hardness then drops sharply if there is no deep freezing - e.g. in liquid nitrogen - to convert the increasing amount of retained austenite). The actual cause of the increasing amount of retained austenite and the associated hardness losses are the martensite start and martensite finish temperatures, which decrease with increasing carbon content, to well below room temperature, see Figure 2. The heat treatment for producing martensite is called hardening ( austenitizing and quenching with martensite formation). Hardening is combined with tempering (first tempering stage up to 200 ° C to remove the glass hardness). The hardenability of a steel can be indicated by the ideal critical diameter .
The martensite start temperature
The temperature at which the martensitic transformation begins is below the equilibrium temperature T 0 , at which austenite and martensite of the same composition have identical free energies G. This fact is shown schematically in Figure 3, assuming a linear GT relationship.
The undercooling below T 0 provides the free enthalpy ΔG (T 0 -MS) for the lattice shear that occurs, for the newly emerging boundary surfaces and the lattice disturbances generated. The austenite-martensite transformation stops when the martensite finish temperature M f is reached . T 0 and thus also M S and M f depend heavily on the alloying elements.
Since the energetically more favorable cementite (Fe 3 C) cannot arise due to the lack of diffusion, the formation of the energetic martensite begins as soon as M S is undershot. With further cooling, an amount of martensite proportional to the subcooling arises, which reaches 100% at M f . Seen clearly, the unconverted austenite is deformed more and more by the martensite formation, so that an ever higher driving force and thus undercooling is necessary to continue the conversion. Then it can also be explained that if M f is below room temperature, a corresponding amount of retained austenite remains, which can only be further converted by deep freezing. From around 1.5% C, the retained austenite can no longer be converted even by freezing in liquid nitrogen. In contrast, a diffusion-controlled transformation by tempering is always possible.
The following table provides an overview of method used to calculate M S . Most approaches assume a linear additive influence of the alloying elements on the martensite start temperature. In fact, there is a coupled influence of the alloying elements, as approach 8 in the table for carbon takes into account.
| linear: M S = M S, 0 -Σf leg (Ma-%) leg [° C] | |||||||||||
| No. | Quote | year | M S, 0 | f leg | C. | Si | Mn | Cr | Mon | Ni | W. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1944 | 499 | f leg = | 317 | 11 | 33 | 28 | 11 | 17th | 11 | |
| 2 | 1946 | 499 | f leg = | 333 | 11 | 33 | 28 | 11 | 17th | 11 | |
| 3 | 1946 | 538 | f leg = | 361 | - | 39 | 39 | 28 | 19th | - | |
| 4th | 1946 | 499 | f leg = | 300 | 11 | 33 | 22nd | 11 | 17th | - | |
| 5 | 1956 | 561 | f leg = | 474 | - | 33 | 17th | 21st | 17th | - | |
| 6th | 1965 | 539 | f leg = | 423 | - | 30.4 | 12.1 | 7.5 | 17.7 | - | |
| 7th | [4] | 1971 | 550 | f leg = | 350 | 5 | 40 | 20th | 10 | 17th | 8th |
| non-linear: MS = M S, 0 - {Σf leg, 1 (Ma-%) leg + Σf leg, 2 (Ma-%) leg, 2 (Ma-%) C } [° C] | |||||||||||
| No. | Quote | year | M S, 0 | f leg, 1/2 | C. | Si | Mn | Cr | Mon | Ni | W. |
| 8th | [11] | 1965 | 512 | f leg, 1 = | 453 | - | - | -15 | 9.5 | 16.9 | - |
| f leg, 2 = | -217 | - | 71.5 | 67.6 | - | - | - | ||||
Residual austenite content
Figure 2 shows the dependence of M S and M f on the carbon content of unalloyed steels. If M f falls below the quenching temperature T u (e.g. room temperature), retained austenite remains in the transformation structure, which is based on the empirical relationship
can be described. B is a temperature-dependent constant: B (20 ° C) = 1.1 · 10 −2 (° C) −1 and B (-196 ° C) = 7.5 · 10 −3 (° C) −1 . In reality , as shown in Figure 4, M S is also dependent on the cooling rate.
Microstructure
The martensitic conversion of the face-centered cubic (motor vehicle) high-temperature phase austenite (γ mixed crystal) into the tetragonal body-centered metastable martensite phase takes place via coordinated lattice shear, whereby the atoms move only over short distances compared to the atomic distance and maintain their proximity relationships. This process can be formally explained using Figure 5. In neighboring unit cells of the austenite lattice, with the lattice constant c A, there are virtual Martensite cells with the dimensions c M '= c A and a M ' = c A √2 / 2. In order to obtain the correct lattice constants of the martensite c M and a M , c M 'has to be reduced by about 20% and a M ' has to be increased by about 12%.
During martensite formation, the octahedral gaps in austenite merge into octahedral gaps in martensite, so that no diffusion of the carbon atoms accommodated in these gaps is necessary. The occupation of the so-called Z-layers of the martensite lattice leads to tetragonal distortion. The ratio a M / c M shows the pronounced dependence on the carbon content shown in Figure 6. According to is quantitative
and
The tetragonism of martensite is influenced in a characteristic way by alloy atoms.
Figure 5 suggests that in the martensitic transformation, the orientation relationship
{111} A → {110} M
<110> A → <111> M
consists. This " Kurdjumow - Sachs relationship" is experimentally confirmed with carbon contents above 0.5 mass percent. Since the stresses arising from the transformation at the austenite-martensite interfaces are relieved by adaptive deformations, the orientation of the habit plane cannot be clearly seen in Figure 5. According to Christian, the habitus levels {111} A , {225} A and {259} A are observed depending on the carbon content .
Since the kfz-γ-mixed crystal is atomically more densely packed than the krz-α-mixed crystal or the trz-martensite, the γ → α-conversion takes place with an increase in volume, which shows the dependence on the carbon content shown in Figure 7. This increase in volume results from a change in length perpendicular to the habitus plane and a shear parallel to it. As explained in Figure 8, the macroscopic shear angle can be determined using surface reliefs that are created on polished surfaces. It is about 10 °.
The adaptive deformations mentioned determine the martensite morphology that forms. The table summarizes the habit levels, orientation relationships and fine structures for unalloyed steels observed with different carbon contents. The massive martensite that forms when the carbon content is low consists of packets of parallel slats within former austenite grains. With higher carbon contents, more and more plate-shaped areas are formed next to the slats, which include areas of retained austenite.
| C content [mass%] | Habitus level | Orientation relationship | Type | Fine structure |
|---|---|---|---|---|
| <0.5 | {111} A or {123} M | Kurdjumow-Sachs | Solid martensite | Packages of parallel battens in <111> M direction. High dislocation density (10 11 to 10 12 cm −2 ) |
| 0.5 to 1.1 | {225} A or {112} M | Kurdjumow-Sachs | Mixed martensite | Side by side: battens (with high dislocation density) and panels (heavily twinned) |
| 1.1 to 1.9 | {225} A or {112} M and {259} A or {111} M | Nishiyama Aquarius | Plate martensite | Arbitrarily arranged lenticular martensite plates and retained austenite, plates twinned, twin planes {112} |
If the martensite formation were to take place via classic homogeneous or heterogeneous nucleation, then, according to Pitsch , such a large amount of energy would be required that cannot be obtained from the existing thermal atomic movements at the low M S temperatures. A thermally activated nucleation of martensite is therefore not possible. It is therefore assumed that so-called preformed nuclei are already present in austenite. With a smaller than the critical size, these can gradually grow due to thermal fluctuation. After reaching the critical size, growth continues unchecked at speeds of up to 5000 m / s.
Another conversion mechanism in which the carbon content of austenite and ferrite does not change is massive conversion. It occurs with very low carbon contents and is based on the rapid movement of incoherent interfaces. After heterogeneous nucleation, growth is diffusion-controlled, and austenite grain boundaries can also be exceeded. Diffusion paths of one to two atomic distances are required for the lattice atoms. The formation temperatures of solid ferrite are higher than those of martensite. An increase in the cooling rate leads to a suppression of the massive transformation in favor of martensite formation. Depending on the formation temperature, one can distinguish between a fast and a slow massive conversion. Near the equilibrium temperature, the interstitial atoms diffuse into the moving interface and thus determine its speed. At lower temperatures, the diffusivity of the interstitial atoms decreases to such an extent that the conversion rate is only limited by the mobility of the interfaces.
Structural modifications of martensite
Depending on the temperature and the alloy content (especially the carbon content), different structural modifications of the martensite arise in the material.
Lath martensite
The lath martensite (also called lath, block or low-carbon massive martensite , in English lath martensite ) is formed at higher temperatures (at temperatures closer to the martensite start temperature) and lower carbon contents of around 0.4–0.5% C, in hypoeutectoid steels. It consists of flattened lancets that are packed close together to form layers and then layer by layer to form massive blocks. It is found predominantly in unalloyed and low-alloy steels with less than 0.4% C, but also in alloys made of iron with <25% nickel. Characteristic is the formation in the form of packages of parallel <1 µm wide lancets without leaving any residual austenite. A structure made of 100% lath martensite only arises if the carbon content is below a maximum of 0.3%.
Lance martensite has a high dislocation density (up to 10 12 cm −2 ) and is significantly more malleable than plate martensite, as it is formed at higher temperatures and can therefore better relieve the elastic tension caused by the folding of the grid through sliding and recovery mechanisms.
Plate martensite
Plate martensite (also called needle-shaped, needle-like, twinned martensite, in English plate martensite or twinned martensite ), is formed at lower temperatures and higher carbon contents of around 0.8–1% C, for example in hypereutectoid steels. The martensite does not grow in a lancet shape, but in a plate shape, in which the plates are not stacked in parallel, but are at different angles to one another. Retained austenite remains in the spaces.
The plates are prevented from growing on the one hand by the grain boundaries of the austenite, and on the other hand by the plates that have already formed at higher temperatures, so that the newly created plates become shorter and shorter over time and wet the space more and more densely. The average length of the plates is between a quarter and a third of the original austenite grain size.
Plate martensite is less malleable than lancet martensite because at lower temperatures the primary mechanism of plastic deformation is not sliding and recovery processes, but rather the formation of twins .
Mixed martensite
In the area between the lancet martensite and the plate martensite, i.e. between about 0.5-0.8% C, an intermediate form, the mixed martensite, arises.
Martensite in chrome-nickel steels
The following methods are possible for the martensitic transformation of CrNi steels:
- a) Austenite γ → martensite α ′ (krz)
- b) Austenite γ → martensite ε (hdp)
- c) Austenite γ → martensite ε (hdp) → martensite α ′ (krz)
In case a), the martensite is created, as described for the pure carbon steels, through cooperative lattice shear.
In case b) the krz lattice is converted into the hdp lattice by shearing individual atomic layers . The orientation relationship of the two grids is:
For case c) there is then a shear in the direction of the normal martensite lattice. The following orientation relationship exists between the ε and the α 'lattice:
Mason diagram
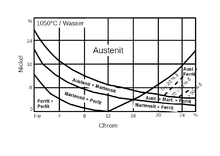
For chrome-nickel steels, the structure quenched after solution annealing is described by the Maurer diagram (Figure 9). With increasing chromium and nickel contents, the structure first becomes pearlitic-ferritic, then martensitic-pearlitic, then austenitic-martensitic and finally purely austenitic.
Schaeffler diagram
With higher chromium contents, delta ferrite occurs, which represents an undesirable structure, especially during welding, the possible formation of which can be assessed in the Schaeffler diagram (Figure 10). The stabilizing effect of other alloy elements on the ferrite or austenite is also taken into account here by combining them into Cr or Ni equivalents, depending on their effect.
Cr equivalent =% Cr +% Mo + 1.5% Si + 0.5% Nb + 2% Ti
Ni equivalent =% Ni + 30% C + 0.5% Mn
Increasing Cr equivalents initially lead to an austenitic-martensitic structure and then to austenite with a very high proportion of delta ferrite. Increasing Ni equivalents work in the opposite direction and reduce the delta ferrite until a purely austenitic structure is formed.
Martensite in titanium and titanium alloys
Pure titanium (element) can exist in two different crystal modifications. Above 882 ° C as high temperature phase β-titanium in the short crystal lattice and below 882 ° C as hexagonal (hdp) α-titanium. When alloying elements are added, a mixed crystal region is formed, a distinction being made between elements that stabilize the α region (Al, Sn, Zr, O, N) and those that stabilize the β region (Mo, Fe, V, Cr, Nb ). See Figure 11.
Formation of Ti martensite
When quenching from the β area in water or oil to temperatures of the α area, martensite-typical diffusion-free lattice shear can occur. Since titanium martensite, unlike steel, does not contain any compulsorily dissolved alloying elements, there is no solidification. The material properties of the titanium alloys can, however, be influenced by setting the structure. For example, it can be quenched from the α + β area and, by subsequent tempering, a fine structure with rounded β-lamellae can be set, which has favorable strength values.
Application examples
Today, sheet metal containing martensite is also used in automotive engineering . In general, one speaks here of multi-phase steels . Specifically, reference is made here to dual-phase steels , TRIP steels and martensitic steels . These are characterized by high strength and can still be formed relatively easily.
Martensite is also used in tool steels , especially in maraging steels .
The formation of martensite structures can be observed very nicely during the differential hardening of Japanese Tamahagane steel in katanas .
Web links
- Video: Martensitic transformation of chrome-nickel steel - MS point 255 ° C . Institute for Scientific Film (IWF) 1967, made available by the Technical Information Library (TIB), doi : 10.3203 / IWF / E-1151 .
- "High-frequency cinematographic recordings at high and low temperatures." (1966) - Image from the collection of the Federal Institute for Scientific Film (ÖWF) in the online archive of the Austrian Media Library
Individual evidence
- ^ NF Kennon, DP Dunne, C. Middleton: Aging Effects in Copper-Based Shape Memory Alloys. In: Metallurgical Transactions. A vol 13A, 1982, pp. 671-673.
- ^ E. Hornbogen: On the Martensite Start Temperature MS. In: Zeitschrift für Metallkunde. Volume 75/10, 1984, pp. 741-746.
- However, ↑ is not yet tempering, as tempering only starts from the 3rd tempering stage 400–600 ° C.
- ↑ Hans-Jochem Steim : Investigations into the hardening behavior of martensite-hardenable steels . Dissertation . University of Karlsruhe, 1970.
- ↑ E. Hesse, HJ Eckstein: Contribution to the transformation behavior of low-carbon unalloyed steels. In: Freiberg research books. VEB-Verlag for the basic industry, 1976, pp. 30-44.
- ^ E. Hornbogen: On the Martensite Start Temperature MS. In: Zeitschrift für Metallkunde. Volume 75/10, 1984, pp. 741-746.
- ^ GS Ansell, SJ Donachie, RW Messler jun .: The Effect of Quench Rate on the Martensitic Transformation in Fe-C Alloys. In: Metallurgical Transactions. 2, 1971, pp. 2443-2449.
- ^ Krauss: Principals of Heat Treatment. American Society for Metals, Ohio 1980.
- ↑ P. Payson, CH Savage: Martensite Reactions in Alloy Steels. In: Transactions of the American Society for Metals. Vol 33, 1944, pp. 261-275.
- ^ ES Rowland, SR Lyle: The Application of MS Points to Case Depth Measurements. In: Transactions of the American Society for Metals. Vol 37, 1946, pp. 27-47.
- ^ RA Grange, HM Stewart: The Temperature Range of Martensite Formation. In: Transactions of the American Society for Metals. Vol 167, 1946, pp. 467-490.
- ↑ AE Nehrenberg: Transactions of AIME. Vol 167 (1946) 494-498.
- ^ W. Steven, AG Haynes: The Temperature of Formation of Martensite and Bainite in Low-alloy Steel. In: JISI. Vol 183, 1956, pp. 349-359.
- ↑ KW Andrews: Empirical Formulas for the Calculation of Some Transformation Temperatures. In: JISI. Vol 302, 1965, pp. 721-727.
- ↑ DP Koistinen, RE Marburger: A General Equation Prescribing the Extent of the Austenite-Martensite-Transformation in Pure Iron Carbon-Alloys and Plain Carbon Steels. In: Acta Metallurgica. 7, 1959, pp. 59-60.
- ^ JM Moyer, GS Ansell: The Volume Expansion Accompanying the Martensite Transformation in Iron-Carbon Alloys. In: Metallurgical Transactions. A Vol 6A, 1975, pp. 1785-1791.
- ↑ EC Bain: The Nature of Martensite Trans. In: AIME, Steel Div. 70, 1924, pp. 25-46.
- ^ BA Bilby, JW Christian: Crystallography of Martensitic Transformations. In: JISI. Vol. 197, 1961, pp. 122-131.
- ↑ a b G. V. Kurdjumov, G. Sachs: About the mechanism of steel hardening. In: Z. Phys. 64, 1930, pp. 325-343.
- ↑ CS Roberts: Effect of Carbon on the Volume Fraction and Lattice Parameters of Retained Austenite and Martensite Trans. In: AIME. 197, 1953, p. 203.
- ^ GV Kurdjumov, AG Khachaturyan: Nature of Axial Ratio Anomalies of the Martensite Lattice and Mechanism of Diffusionless γ → α Transformation. In: Acta Metallurgica. 23, 1975, pp. 1077-1088.
- ^ JW Christian: Transformations in Metals and Alloys An International Series on Materials Science and Technology. Pergamon Press Oxford, New York / Frankfurt 1965.
- ↑ E. Macherauch, O. Vöhringer: Deformation behavior of hardened steels. In: Härterei Technische Mitteilungen. 41/2, 1986, pp. 71-91.
- ↑ W. Pitsch: Martensitumwandlung Basics of the heat treatment of steel. Verlag Stahleisen, Düsseldorf 1976, pp. 79-91.
- ↑ M. Cohen: Operational Nucleation in Martensitic transformation. In: Metallurgical Transactions. 3, 1972, pp. 1095-1098.
- ^ HJ Eckstein: Heat treatment of steel. VEB German publishing house for basic industry, Leipzig 1971, pp. 199-214.
- ↑ J. Singh: Slow Massive Transformation in Fe and Fe-Ni-Alloys. In: Scripta Metallurgica. Vol 20, 1986, pp. 173-176.
- ^ E. Folkhard: Metallurgy of the welding of stainless steels, Springer-Verlag, Vienna 1984.
- ^ H. Schumann: Metallography. 13th edition. German publishing house for basic industry, Leipzig 1991.
- ^ V. Schoss: Martensitic transformation and fatigue of austenitic stainless steels, structural changes and possibilities of early detection of fatigue damage, dissertation TU Freiberg, 2001.
- ↑ J. Rösler, C. Siemers: Specialist laboratory for titanium and titanium alloys. TU Braunschweig, 2007.






![{\ displaystyle RA = \ exp [-B (M_ {S} -T_ {U})]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3a8418fc071b37c73f83909069babde86bac44a9)



![{\ displaystyle \ alpha _ {M} = (2 {,} 861-0 {,} 013 \, {\ text {Mass -}} \% \ mathrm {C}) 10 ^ {- 8} [cm]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f8009e7d6671cab76a145a06d1cb0dbc05af64c4)
![{\ displaystyle c_ {M} = (2 {,} 861 + 0 {,} 116 \, {\ text {Mass -}} \% \ mathrm {C}) 10 ^ {- 8} [cm]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4ccdaa767c4ba603f7f152b67102ec31f15408a0)


![[10 \ overline 1] _ {\ gamma} \ | [11 \ overline 20] _ {\ epsilon}](https://wikimedia.org/api/rest_v1/media/math/render/svg/46dcf78019c50866015fb6b87dc6477098f9251b)

![[11 \ overline 20] _ {\ epsilon} \ | [11 \ overline 1] _ {{\ alpha \ prime}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7d1aa2c22403b89473bf10dbf61ab0ba8a29c059)
