Physical vapor deposition
The term physical vapor deposition ( English physical vapor deposition , shortly PVD ), rarely physical vapor deposition refers to a group of vacuum-based coating processes and thin film technologies . In contrast to chemical vapor deposition processes , physical processes are used to convert the starting material into the gas phase . The gaseous material is then fed to the substrate to be coated , where it condenses and forms the target layer.
Procedure
The technologies listed below as well as reactive variants of these processes belong to the group of methods of physical vapor deposition.
- Evaporation process
- Thermal evaporation (also steaming called)
- Electron beam (Engl. Electron beam evaporation )
- Laser beam vaporization ( pulsed laser deposition , pulsed laser ablation ): Atoms and ions are vaporized by a short, intense laser pulse.
- Arc evaporation (engl. Arc evaporation , Arc-PVD ) atoms and ions by a strong stream of converted in an electrical discharge between two electrodes flows (as in a flash), removed from the starting material and in the gas phase.
- Molecular beam epitaxy (Engl. Molecular beam epitaxy )
-
Sputtering (sputter deposition, sputtering): The starting material is sputtered by ion bombardment and transferred to the gas phase.
- Ion beam-assisted deposition (Engl. Ion beam assisted deposition , IBAD )
- Ion plating
- ICB technology ( ionized cluster beam deposition , ICBD )
General process description
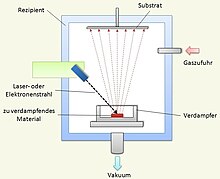
What all these processes have in common is that the material to be deposited is in solid form in the mostly evacuated coating chamber. When bombarded with laser beams, magnetically deflected ions or electrons, as well as arc discharge , the material, which is known as the target , is vaporized. The proportion of atoms , ions or larger clusters in the steam varies from process to process. The vaporized material moves through the chamber either ballistically or guided by electrical fields and hits the parts to be coated, where a layer is formed.
In order for the vapor particles to reach the components and not be lost due to scattering on the gas particles, it is necessary to work in a vacuum. Typical working pressures are in the range from 10 −4 Pa to approx. 10 Pa. Since the vapor particles spread in a straight line, areas that are not visible from the location of the vapor source are coated at a lower coating rate. If all surfaces are to be coated as homogeneously as possible, the parts must be moved in a suitable manner during the coating. This is usually done by rotating the substrate.
If the vapor particles hit the substrate, they begin to be deposited on the surface through condensation. The particles do not stay where they hit the substrate, but move, depending on how high their energy is, along the surface ( surface diffusion ) in order to find an energetically more favorable place. These are places on the crystal surface with as many neighbors as possible (higher binding energy ).
In order to increase the coating rate and layer homogeneity , the systems are slightly varied depending on the coating process and the material to be deposited. For example, during thermal evaporation, a negative voltage ( bias voltage ) is applied to the parts to be evaporated . This accelerates the positively charged vapor particles or metal ions (see corresponding article).
Since processes for physical vapor deposition are vacuum coatings, they are mostly operated in batch operation in production: charging (filling) the vacuum chamber, evacuation, coating, ventilation, opening and removal of the coated parts. For certain applications (coating of sheet metal, fibers or wires and architectural glass), however, there are continuous systems in which the negative pressure is achieved via a lock system and the material to be coated is fed in continuously.
With some PVD processes (magnetron sputtering, laser beam evaporation, thermal evaporation, etc.), very low process temperatures can be achieved. This makes it possible to coat even low-melting plastics. The “ eggshell effect ” previously feared when coating plastics , i.e. H. Crack formation and detachment of the layer due to the excessive resilience of the substrate in the event of punctual loading could also be minimized by targeted influence on the layer structure with multilayer coatings with a biomimetic structure similar to that of mussel shells.
layers
With the various PVD variants, almost all metals and also carbon can be deposited in a very pure form. If reactive gases such as oxygen , nitrogen or hydrocarbons are fed into the process, oxides , nitrides or carbides can also be separated.
Processes for physical vapor deposition are mainly used for the deposition of thin layers in the range of a few nanometers up to a few micrometers . The internal stresses within the layers also increase with the layer thickness , which can lead to detachment from the substrate ( delamination ). This is one of the reasons why the PVD process cannot easily produce layers of any thickness. Research results of the Fraunhofer Institute IWS in Leipzig show possibilities of applying layer thicknesses of> 20 µm with the PVD process. Many layers in the nm range are applied one on top of the other.
Applications
Physical vapor deposition layers are used in many areas of industry.
Surface reinforcement
Tools made from coated cutting materials are now mostly used, especially in the machining sector. Hard coatings based on titanium nitride (TiN), titanium carbonitride (TiCN) or titanium aluminum nitride (TiAlN) are mainly used as coatings today . As early as the early 1990s, various research institutions were investigating further possible uses in the area of tool coatings for die casting of aluminum and magnesium. Chromium- based coating systems such as chromium nitride (CrN), chromium vanadium nitride (CrVN) and chromium aluminum nitride (CrAlN) are mainly used in these applications . Coatings made of CrN are also often used for corrosion protection.
microelectronics
PVD layers are used in microelectronics z. B. used to produce metal or (organic) semiconductor layers. In the entertainment electronics sector, data carriers such as hard drives, CDs and DVDs are coated using the PVD process.
surface protection
Polyethylene films in the food industry (e.g. bags of potato chips) have a thin PVD layer on the inside as a vapor barrier. In many other applications of plastics (e.g. for wear protection, for optical and decorative purposes), PVD coating processes at low temperatures (room temperature) are increasingly used. Architectural glass or displays are also covered with protective layers using the PVD process.
Fuel cells
In fuel cells , in particular solid oxide fuel cells , the electrolytes can be generated by PVD in order to obtain the thinnest possible electrolytes that increase the electrical performance of a cell.
Fuel assemblies
A more recent application is in the manufacture of nuclear fuel elements . Before cladding, a barrier layer made of z. B. zirconium on the uranium-molybdenum foils (U-Mo) vapor-deposited. This prevents unwanted diffusion between the fuel (U-Mo) and the fuel element casing (aluminum) during operation.
literature
- Gerhard Kienel, Klaus Röll: Vacuum coating: Volume 2 processes and systems . Springer, 1997, ISBN 3-540-62266-7 .
- Donald M. Mattox: Handbook of Physical Vapor Deposition (PVD) Processing . 2nd Edition. William Andrew, 2010, ISBN 978-0-8155-2037-5 (English).
- KS Sree Harsha: Principles of Physical Vapor Deposition of Thin Films . Elsevier Science & Technology, 2006, ISBN 0-08-044699-X (English).
- Christoph Eisenmenger-Sittner: Physics and Technology of Thin Films; Coating process. (PDF; 942 kB) Vienna University of Technology; Institute for Solid State Physics, pp. 1 - 38 , accessed on January 6, 2020 .
Individual evidence
- ↑ Dr. Ottmar Zimmer: Hard layers> 20 μm - new possibilities for thin-film technology. (PDF; 107 kB) IWS Fraunhofer, 2008, accessed on January 6, 2020 .
- ↑ PVD coating. Retrieved January 6, 2020 .
- ↑ Technical University of Munich: Fuel production for the FRM II. Accessed on January 6, 2020 .