Solid oxide fuel cell
The solid oxide fuel cell ( English solid oxide fuel cell , SOFC ) is a high temperature fuel cell that is operated at an operating temperature of 650-1000 ° C. The electrolyte of this cell type consists of a solid ceramic material that is able to conduct oxygen ions but has an insulating effect on electrons. Many solid oxide fuel cell projects are still in development , some are already on the market.
Terms
The interconnection of several solid oxide fuel cells is known as an SOFC stack . SOFC systems also include the heat exchanger , the reformer , the direct current-alternating current inverter , the control system and other technology that is necessary for operation.
principle
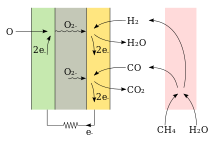
The solid electrolyte is designed as a thin membrane in order to be able to transport the oxygen ions with little energy; this requires high temperatures. Gas-permeable electrical conductors are attached to the sides of the electrolyte as a cathode and anode . The outer side of the cathode facing away from the electrolyte is surrounded by air, the outer anode side by fuel gas. Unused air and unused fuel gas as well as combustion products are extracted.
The function of every galvanic cell is based on a redox reaction in which reduction and oxidation take place spatially separately, namely at the interface between electrode and electrolyte . In the SOFC, this redox reaction is a reaction of oxygen with the fuel, e.g. B. hydrogen or carbon monoxide . There is excess oxygen on the cathode side, while there is a lack of oxygen on the anode side because the oxygen present is equal to z. B. reacts with the hydrogen. This concentration gradient causes the oxygen to diffuse from the cathode to the anode. The electrolyte in between is only permeable to oxygen ions . Once the oxygen molecule (O 2 ) has reached the interface between the cathode and the electrolyte, it breaks down into two oxygen ions, each with a double negative charge, absorbing four electrons and can thus penetrate the barrier (ion conductor). At the anode, two hydrogen molecules (H 2 , fuel gas) are converted into four single-positive hydrogen ions, releasing a total of four electrons. When they reach the boundary with the anode, the two oxygen ions react catalytically with the four single-positive hydrogen ions, releasing heat and the corresponding combustion products. The prerequisite for the ionization of hydrogen and oxygen at the respective electrode is a current flow, the purpose of the solid oxide fuel cell, which can be used for other purposes.
At a voltage of around 0.7 V, a power density of around 1 W / cm² (corresponding to a current density of 1.4 A / cm²) is achieved. For fuel gases and the corresponding reaction equations, see section #Reaction equations .
Designs
Basically, a distinction is made based on the design: tubular (tubular, see Fig.) And planar (flat) SOFCs. Tubular SOFCs offer technical advantages in sealing the electrode gas spaces from one another. Planar SOFCs are more suitable for applications that require high volumetric power densities. Due to the load-bearing structure, a distinction is still made between electrolyte-supported and anode-supported SOFC. The use of 3D printers , the ink of which consists of ceramic particles and solvents , makes it possible to realize different designs.
Technical challenges
Due to the high operating temperature, it is possible to use less noble, i.e. less expensive, materials than, for example, with the PEMFC and at the same time to achieve high power densities and high efficiencies . However, the high operating temperature is also the reason for almost all technical challenges.
The sealing technology between the gas spaces is very complex ( high temperature seal ). Conventional flat seals simply fail. Cohesive connections can short-circuit the electrodes. Therefore, special sealing materials such as glass solders are currently used for SOFC applications.
Mechanical stresses during operation are mainly due to temperature differences in the cell and different thermal expansion coefficients (TEC) of the materials. In addition, there is an increased tendency to creep or oxidation processes or high temperature corrosion .
development
Westinghouse Power Corporation (now Siemens Power Generation ) took the first big step towards a marketable SOFC in the late 1980s. She built a functional prototype with an electrolyte made from yttrium- stabilized zirconium oxide , or YSZ for short. A method has been adapted, the electrochemical vapor deposition , which can apply thin layers on porous material, e.g. B. for the production of the anode. The tubular arrangement of the cells also eliminates the need for seals. The efficiency was good, but the vapor deposition process was expensive and the cells were still quite large for their performance.
In the mid-1990s, Tatsumi Ishihara found a new electrolyte material, lanthanum gallium oxide doped with strontium and magnesium, or LSGM for short. It made a major contribution to the development of SOFCs with lower temperatures. They got the designation IT -SOFCs , English intermediate-temperature SOFCs, or medium-temperature SOFCs. This allows ceramic parts to be replaced by metals, e.g. B. the mechanical connections between the cells.
materials
electrolyte
SOFCs use electrolytes made of solid oxide ceramics , typically yttrium-stabilized zirconium oxide, more precisely yttrium oxide partially stabilized or yttrium oxide - fully stabilized zirconium dioxide . Alternatively, strontium- and magnesium-doped lanthanum gallium oxide (LSGM) or, rarely, cerium oxide doped with gadolinium, is used.
To produce a thin electrolyte layer from YSZ, a coating with fine YSZ dust is applied to an electrode and sintered . As mentioned, the coating can be carried out by electrochemical vapor deposition . If necessary, several layers can also be co-sintered, for example the electrolyte and an electrode. Ceramics, e.g. B. an electrode, can also be prefabricated by film casting and firing and then co-sintered with other layers in a sufficiently hot oven.
cathode
The cathode of a YSZ electrolyte is classically made of the ceramic material lanthanum strontium manganite, (La, Sr) MnO 3 , or LSM for short . It can also be mixed with yttrium-stabilized zirconium oxide (YSZ) in order to influence the expansion coefficient. Especially for low-temperature SOFCs, lanthanum strontium cobaltite, (La, Sr) CoO 3 , LSC , the same with iron, LSF , or the combination of LSCF are possible. However, a separating layer consisting of a cerium compound to the YSZ electrolyte seems necessary to counteract harmful chemical processes.
The cathode of an LSGM electrolyte is usually made of Samarium Strontium Cobaltite (Sm, Sr) CoO 3 , SSC for short.
anode
The anode is made entirely of nickel cermet ( English ceramic-metal ), a composite material made of the metal nickel and the ceramic YSZ, rarely Sc SZ or a cerium oxide .
Reaction equations
Theoretically, fuel cells can implement any sufficiently reactive redox reaction if the starting materials are liquid or gaseous. Solid fuels have to be converted into liquids or gases either separately or in the fuel cell. For example, coke can be used in a suitable SOFC at a sufficiently high temperature, since it can be converted into carbon monoxide with the reaction product CO 2 .
In practice, the focus is mostly on fuels that are readily available. In the case of SOFCs, these are primarily the gases hydrogen and synthesis gas . Hydrogen is very reactive and can be easily produced, especially in connection with carbon monoxide as in synthesis gas. Thus, natural gas , crude oil distillates, or even wood chips for SOFCs are processed. The oxidizing agent is the oxygen in the air.
| Reaction equations 1 | Reaction equations 2 | |
|---|---|---|
| anode |
Oxidation / electron donation |
Oxidation / electron donation |
| cathode |
Reduction / electron uptake |
Reduction / electron uptake |
| Overall reaction |
Redox reaction / cell reaction |
Redox reaction / cell reaction |
The internal charge transport takes place by means of O 2− ions. The SOFC needs oxygen on the cathode side and produces water and / or CO 2 on the anode .
Operating the solid oxide fuel cell directly with methane leads to problems with the pyrolysis of CH 4 and the resulting carbon deposits.
| anode |
Oxidation / electron donation |
|---|---|
| cathode |
Reduction / electron uptake |
| Overall reaction |
Redox reaction / cell reaction |
Efficiency
In contrast to heat engines with a downstream generator such. B. Gas-fired power plants , which convert the chemical energy into heat, power and then into electricity, the electricity (in addition to heat) is generated directly in fuel cells. This means that their efficiency is not limited by the thermodynamic limits of the Carnot process , and the theoretical efficiency is very high. One reason for the practical limits of the achievable efficiencies is that, for the optimal use of the relatively expensive fuel cells, minimum current densities are sought, which in turn require an adequate supply of fuel and removal of the exhaust gases. Since the combustion products at the anode (e.g. CO 2 and / or H 2 O) occur at the same point where the fuel gases (H 2 , CH 4 , CO) are required, mixing is difficult to avoid. It is true that the gas flows could be slowed down or the electrode area increased in order to make the conversions more complete and the efficiencies higher, but the current density and the output of the cell would then be too low. Therefore, the fuel gas is blown in so that part of it comes out of the cell unburned. The corresponding energy reduces the efficiency of the cell.
This application is particularly interesting for the power-to-gas process, which has only relatively low levels of efficiency with conventional technology. With reversibly operated solid oxide fuel cells, however, electricity-to-electricity efficiencies of up to about 70% are possible, which means that the degree of efficiency is roughly comparable to that of pumped storage power plants . The reverse SOFC is the SOEC .
literature
- Sven Geitmann: Hydrogen & Fuel Cells - The Technology of Tomorrow. 2nd Edition. Hydrogeit Verlag, Kremmen 2004, ISBN 3-937863-04-4 .
- Manuel Ettler: Influence of reoxidation cycles on the operational stability of anode-supported solid oxide fuel cells . Forschungszentrum Jülich, 2008, ISBN 978-3-89336-570-8 , p. 120 ( limited preview in Google Book search).
Web links
- Fuel Cell Handbook (Seventh Edition) from page 7–1, extensive scientific overview, company EG&G Technical Services, Inc. (English, PDF, 5 MiB)
- MeMO - Electrochemical metal-metal oxide high-temperature storage for centralized and decentralized stationary applications , presentation graphics of an ongoing project, Forschungszentrum Jülich, accessed 2014
- SOFC Status and Challenges Presentation of a SOFC with pictures, Chris De Minco & Dr. Subhasish Mukerjee, Delphi Automotive Systems, 2001
Individual evidence
- ↑ Development status and future prospects for residential and commercial use of SOFC systems (English), Minoru Suzuki from Osaka Gas Co. Ltd. - SOFC manufacturers are for example the company Osaka Gas Co. Ltd. itself, in Japan, it makes a 700 watt SOFC, the company Ceres Power, GB, also 700 watt, or the company Bloom Energy, USA, a 100 kW device.
- ↑ Alberto Varone, Michele Ferrari, Power to liquid and power to gas: An option for the German Energiewende . In: Renewable and Sustainable Energy Reviews 45, (2015), 207–218, p. 209, doi : 10.1016 / j.rser.2015.01.049 .
- ↑ a b 3D-Printed Solid Oxide Fuel Cells from High Particle Content Liquid Inks , accessed December 9, 2014.
- ↑ Tatsumi Ishihara, Hideaki Matsuda, Yusaku Takita. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. J.Am. Chem. Soc. 116: 3801-3803. 1994
- ↑ Synthesis and characterization of ceramic layers made of (La, Sr) (Ga, Mg) O3-x , Thorsten Maldener
- ↑ Fuel Cell Handbook (Seventh Edition) from page 7–1, extensive scientific overview, company EG&G Technical Services, Inc. (English, PDF, 5 MiB), ISBN 978-0-387-77707-8 , 2009
- ↑ Low Cost Fabrication Processes for Solid Oxide Fuel Cells Section Cathode Tape Production, Presentation, MM Seabaugh .., NexTech Materials Ltd., 2000. Process for YSZ layer on LSM substrate.
- ↑ Function of the high-temperature fuel cell ( memento of the original from November 5, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , short introduction, world of physics
- ↑ Introduction to high-temperature fuel cells (SOFC) , short introduction, Institute for Technical Thermodynamics of the German Aerospace Center
- ↑ Perovskite Oxide for Solid Oxide Fuel Cells Chapter 2: Overview of Intermediate-Temperature Solid Oxide Fuel Cells, page 28, extensive scientific overview, by Tatsumi Ishihara (publisher), Chapter 2 by Harumi Yokokawa, Springer, 2009
- ^ AJ Appleby, FR Foulkes, Fuel Cell Handbook, Van Nostrand Reinhold, New York, NY, 1989. Quoted in: Fuel Cell Handbook, EG&G
- ↑ a b Biomass CHP technology for the medium output range, based on solid fuel gasification technology and a solid oxide fuel cell (SOFC). In: Research> ongoing projects. Wuppertal Institute for Climate, Environment, Energy gGmbH, accessed on April 23, 2019 .
- ↑ Yong-min Xie, Jiang-lin Li, Jin-xing Hou, Pei-jia Wu, Jiang Liu: Direct use of coke in a solid oxide fuel cell . In: Journal of Fuel Chemistry and Technology . tape 46 , no. 10 , October 2018, p. 1168-1174 , doi : 10.1016 / S1872-5813 (18) 30048-3 .
- ↑ Jensen et al., Large-scale electricity storage utilizing reversible solid oxide cells combined with underground storage of CO2 and CH4 . In: Energy and Environmental Science (2015), doi : 10.1039 / c5ee01485a .