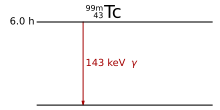Decay scheme
The decay scheme of a radioactive atomic nucleus is a graphic representation of the energy relationships during decay, which can be complicated.
A simple example is the decay of radioactive cobalt -Isotops 60 Co . 60 Co changes to an excited state of 60 Ni with the emission of an electron ( beta decay ) with a half-life of 5.26 years , which reaches the ground state very quickly via two gamma transitions.
Many decay schemes can be found in the Table of Isotopes .
It is useful to the image in a coordinate system to present, where the abscissa represents the atomic number , the energy of the nuclear states is plotted on the ordinate. The arrows indicate the emitted (= emitted) particles; vertical arrows indicate gamma transitions, the oblique arrow a beta transition. In the case of the gamma transition , the gamma energy is given, in the case of beta decay the maximum energy of the emitted electrons. Gamma radiation is mostly emitted after a beta decay, it arises almost immediately after the beta decay (exception see below).
Nickel is to the right of cobalt because the atomic number of nickel is 1 greater than that of cobalt: the atomic number increases by 1 during beta decay. In the case of a positron decay , in which the atomic number decreases, the oblique arrow would run from right to left, also at an alpha decay (see below).
Since energy is a conserved quantity and high-energy radiation is emitted when the nucleus decays, arrows can only run (vertically or diagonally) from top to bottom.
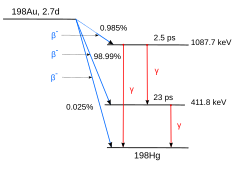
A somewhat more complicated decay scheme is that of the gold isotope 198 Au, which is obtained by neutron irradiation of natural gold in the nuclear reactor . 198 Au decays through beta decay to excited states (or to the ground state) of the mercury isotope 198 Hg. In the picture, mercury is to the right of gold because gold has an atomic number 79, mercury an atomic number 80.The excited states decay after a very short time (2.5 or 23 ps; 1 picosecond is a trillionth of a second) also to the ground state.
While excited nuclear states are mostly very short-lived and only occur as a result of beta decay (see above), the excited state of the technetium isotope shown here on the right is “metastable” (hence the “m” in 99m Tc), i.e. i.e., relatively durable. It disintegrates by means of gamma radiation with a half-life of 6 hours.
Here an alpha decay is shown on the left , namely that of the element polonium discovered by Marie Curie with the mass number 210. The isotope 210 Po is the penultimate member of the uranium-radium decay series ; it decays to a stable lead isotope with a half-life of 138 days . In almost all cases, the decay takes place via the emission of alpha radiation of 5.305 MeV . Only one in 100,000 cases does an alpha particle of lower energy appear; the decay leads to an excited state of the 206 Pb, which again leads to the ground state via gamma radiation.
A decay scheme can also be much more complicated than the one shown here. 20 or more possible states (levels) with a large number of possible transitions are not uncommon. The emitted gamma radiation then forms a spectrum with a correspondingly large number of different energies (spectral lines).
Individual evidence
- ↑ Claus Grupen: Basic course on radiation protection . Vieweg, 1998, ISBN 978-3-528-06949-0 , page 15
- ^ CM Lederer, JM Hollander, I. Perlman: Table of Isotopes, Wiley (1968)