Allotropy
As Allotropie (. V . Greek : ἀλλοτροπέω - change or ἀλλοτρόπως - in a different way ) is referred to the appearance, when a chemical element in the same aggregate state in two or more structural forms occurs, which physically and in their chemical reactivity different from each other . Allotropies are also known as modifications of a chemical element in chemistry, mineralogy, and materials science .
The term comes from Jöns Jakob Berzelius . The phenomenon was already demonstrated by Antoine de Lavoisier when he showed that diamond was made of pure carbon.
Examples
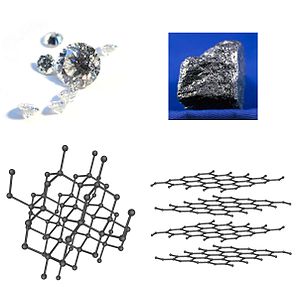
- Carbon occurs both as colorless, extremely hard diamond , which is an electrical insulator , and as black, soft graphite , whichconducts electrical current , and as macromolecules fullerene , carbon nanotubes , cyclo [18] carbon and graphene .
- White phosphorus is highly toxic and self-igniting, whereas red and black phosphorus are non-toxic and require activation to burn .
- The oxygen in the air we breathe (O 2 , dioxygen) is odorless and vital, its allotropic variant ozone (O 3 ) has a pungent smell and is harmful to health even in very small quantities (> 100 ppb).
- Tin has the forms α-Sn and β-Sn. The tin plague occurs when β-Sn is converted to α-Sn.
Further known examples are e.g. B. represent the elements sulfur , selenium , titanium, iron (α-iron, γ-iron etc.) as well as boron , antimony and silicon .
Delimitation of the term
In the case of different isotopes of an element one does not speak of allotropy, since these do not differ or only differ very slightly in their chemical properties.
If a chemical compound occurs in the solid state in several crystal forms (modifications), one speaks of polymorphism .
If two modifications can be mutually converted into one another through temperature or pressure changes, one speaks of enantiotropy . If this transformation occurs only in one direction, while the other modification can only be represented indirectly , this transition is called monotropic (example: polymeric phosphorus ).
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .