Organic aluminum compounds
Organic aluminum compounds , also called organic aluminum compounds , are chemical compounds that bond between carbon and aluminum . They are among the most important organometallic compounds . Exemplary organoaluminum compounds are the dimer trimethylaluminum , the monomer triisobutylaluminum and the titanium-aluminum compound Tebbe reagent . The behavior of organoaluminum compounds is influenced by the polarity of the C-Al bond and the high Lewis acidity of the mostly three- coordinate aluminum atom. Technically, these compounds are used primarily for the production of poly olefins .
history
The first organoaluminum compound (C 2 H 5 ) 3 Al 2 I 3 was discovered in 1859. However, aluminum organyls were of little importance until Karl Ziegler and colleagues discovered the direct synthesis of trialkylaluminum compounds in 1950 and were able to use them for catalytic olefin polymerization. This discovery ultimately led to the awarding of the Nobel Prize to Ziegler.
Structure and relationships
Aluminum (III) compounds
Organoaluminum compounds usually have three- or four-fold coordinated aluminum centers, although higher coordination numbers with inorganic ligands (such as fluoride) are also observed. In line with general trends, four-coordinate aluminum compounds are preferably tetrahedral. Compared to the boron atom, the aluminum atom is larger, so that four carbon ligands can easily be accommodated. For this reason, triorganoaluminum compounds are usually dimers, with a pair of alkyl bridging ligands, for example Al 2 (C 2 H 5 ) 4 (μ-C 2 H 5 ) 2 . Despite the common name triethylaluminum , this compound contains two aluminum centers and six ethyl groups. If the organoaluminum compound contains hydrides or halides, these smaller ligands tend to occupy the bridge sites. Triple coordination occurs when the R groups are bulky, e.g. B. Al (Mes) 3 (Mes = 2,4,6-Me 3 C 6 H 2 or mesitylene ) or isobutyl.
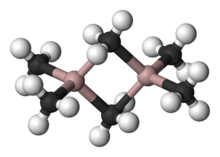
Ligand exchange in trialkylaluminum compounds
Trialkylaluminum dimers are usually subject to a dynamic equilibrium; Through this, they exchange bridging and terminal ligands, as well as the ligands with one another. The methyl exchange is quite rapid even in non-coordinating solvents, as confirmed by proton NMR spectroscopy . The 1H-NMR spectrum of Me 6 Al 2 at −25 ° C consists of 2 signals in a 1: 2 ratio, as is to be expected from the structure. At 20 ° C, on the other hand, only a signal is visible, since the exchange of bridging and terminal methyl groups is too fast to be resolved by NMR spectroscopy. The high Lewis acidity of the monomeric species is related to the size of the Al (III) center and its tendency to reach the octet configuration .
Low-valent organoaluminum compounds
The first organoaluminum compound with an Al-Al bond, (((Me 3 Si) 2 CH) 2 Al) 2 (a dialane), was found in 1988. Compounds of this type are typically obtained by reducing dialkyl aluminum chlorides with metallic potassium:
- (R 2 AlCl) 2 + 2 K → R 2 Al-AlR 2 + 2 KCl
Another notable group of alans are tetraalanes, made up of four Al (I) centers. These compounds have a tetrahedrane core shown by ( Cp * Al) 4 and ((Me 3 Si 3 C) Al) 4 . The cluster [Al 12 ( i-Bu ) 12 ] 2− was found from similar studies on the reduction of organoaluminum compounds. This dianion adopts an icosahedral structure reminiscent of dodecaborate ([B 12 H 12 ] 2− ). Its formal oxidation state is less than one.
presentation
Like organosilicon compounds, organic aluminum compounds are primarily formed by the following three processes:
through oxidative addition of organyl halides to aluminum (in the so-called direct process)
- 2 Al + 3 RX → RAlX 2 / R 2 AlX
through nucleophilic substitution of aluminum halides by organyl ions (metathesis)
- R 2 Al-X + R - → R 2 Al-R + X -
or by inserting alkenes or alkynes into the Al-H bond of organylalanes (hydroalumination):
- R 2 Al-H + H 2 C = CH 2 ⇌ Al-CH 2 -CH 2 -H.
The transmetallation of organyl mercury is rarely used:
- 2 Al + 3 HgR 2 → 2 AlR 3 + 3 Hg.
From alkyl halides and aluminum (direct process)
Simple trialuminum alkyls Al 2 R 6 are produced industrially using the Hüls process . The method is only relevant for compounds with R = Me , Et ( trimethylaluminum and triethylaluminum ). The two-step process begins with the alkylation of aluminum powder:
- 2 Al + 3 EtCl → Et 3 Al 2 Cl 3 ⇌ Et 4 Al 2 Cl 2 + Et 2 Al 2 Cl 4
- Aluminum and chloroethane react to form ethylaluminum chloride, which is in equilibrium with the disproportionation products.
The reaction is reminiscent of the synthesis of Grignard compounds . The product, Et 3 Al 2 Cl 3 , is called ethyl aluminum sesquichloride . “ Sesquichloride ” refers to the average Cl: Al ratio of 1.5. The sesquichloride disproportionates in an equilibrium reaction ; the Et 2 Al 2 Cl 4 is undesirable in the reaction mixture and is complexed and precipitated by adding sodium chloride :
- Et 2 Al 2 Cl 4 + 2 NaCl → 2 Na [EtAlCl 3 ] s ↓.
The Et 4 Al 2 Cl 2 is separated off by distillation and reduced with sodium to the triorganyl aluminum derivative:
- 3 Et 4 Al 2 Cl 2 + 6 Na → 2 Et 6 Al 2 + 6 NaCl + 2 Al.
The overall equation is:
- Al + 3 RCl + 3 Na → AlR 3 + 3 NaCl.
Hydroalumination
In hydroalumination (also known as the Ziegler process), alkenes (here: ethene) react with aluminum and hydrogen. To do this, two individual reactions are combined. First, aluminum, hydrogen and aluminum triorganyls react to form dialkyl aluminum hydride (the "increase"):
- Al + 3/2 H 2 + 2 AlEt 3 → 3 Et 2 AlH
In the next step, these hydrides are reacted with the alkene in a hydroalumination (in the "addition"):
- 3 Et 2 AlH + 3 CH 2 = CH 2 → 3 AlEt 3
The overall equation is:
- Al + 3/2 H 2 + alkene → AlR 3
Since the addition is reversible, numerous other trialkylorganyls can be industrially produced in this way with a subsequent carbaluminization, e.g. B. Tris (n-octyl) aluminum from tris (/ - butyl) aluminum (see chapter dehydro-, hydro- and carbaluminization ).
Laboratory preparation
Since many aluminum organyls are commercially available through industrial production at low cost, production in the laboratory is limited to special compounds. These are mostly produced by metathesis or transmetalation.
Metathesis of aluminum trichloride with RLi or RMgX gives the trialkyl:
- AlCl 3 + 3 BuLi → Bu 3 Al + 3 LiCl
The transmetalation proceeds as follows:
- 2 Al + 3 HgPh 2 → 2 AlPh 3 + 3 Hg
Reactions
The high reactivity of organoaluminum compounds towards electrophiles is attributed to the charge separation between the aluminum and carbon atoms.
Lewis acidity
Organoaluminum compounds are hard acids and easily react with hard bases such as pyridine, THF and tertiary amines. The products are tetrahedral at the Al center.
Dehydro, hydro and carbaluminization
Triorganylalanes AlR 3 easily disintegrate in a dehydroaluminization:
- R 2 Al-CR 2 -CR 2 H ⇌ Al-H + R 2 C = CR2
It is an equilibrium reaction, alkenes can be added to di- or monoorganylalanes (RAlH 2 or R 2 AlH) in a hydroalumination process . The addition is stereoselective ( cis ) and quite regioselective ( anti-Markovnikov ), but not very chemoselective (in addition to CC multiple bonds, bonds to other elements are also attacked; C = Y and C≡Y). The willingness to hydroaluminate grows again:
- RCH = CHR <R 2 C = CH 2 <RCH = CH 2 <CH 2 = CH 2
In addition, triorganyl alanes react in the carbaluminization. An alkene or alkyne is inserted into the Al-C bond:
- R 2 Al-Et + H 2 C = CH 2 → R 2 Al- (CH 2 -CH 2 ) n-Et.
Successive insertions allow long carbon chains to be formed on aluminum (up to 200 carbon atoms). The competition between dehydroaluminization and carbaluminization acts as a limitation; In contrast to hydroalumination, however, carbalumination is not reversible. The rate law of the reaction follows:
- v = k [(Et 3 Al) 2 ] 1/2 * [alkene].
It is assumed that the mechanism for carbaluminization proceeds via the formation of an aluminum organyl-alkene complex, which rearranges to the product in a second step:
Hydroalumination (insertion of an alkene into an aluminum-hydrogen bond) also proceeds according to this mechanism. However, the mechanism is only reversible in the case of hydroalumination.
From a technical point of view, carbaluminization is initially of importance for the production of precursors for biodegradable surfactants H 3 OS – O– (CH 2 –CH 2 ) n –H (with n about 13). The reaction of the aluminum organyl with oxygen initially produces the alcoholates R 2 Al – O– (CH 2 –CH 2 ) n – H and, after reaction with water, long-chain, unbranched alcohols H – O– (CH 2 –CH 2 ) n – H .
Electrophiles
The Al-C bond is so strongly polarized that the carbon is very basic. Acids release alkanes in the reaction. For example, alcohols give alcoholates :
A variety of acids can be formed in this way. Amido derivatives are formed with amines. Trialkylaluminum compounds form dialkylaluminum carboxylates with carbon dioxide :
The transformation is reminiscent of the carbonization of Grignard reagents .
With oxygen one obtains the corresponding alkoxides, which can be hydrolyzed to alcohols:
A structurally characterized organoaluminum peroxide is .
Alkene polymerization
Organoaluminum compounds are used industrially as catalysts for the alkene polymerization of polyolefins , for example the methylaluminoxane catalyst .
properties
The aluminum alkyls are colorless liquids. Aluminum alkyls with short alkyl chains R ignite spontaneously in air and react explosively in contact with water; those with long residues are less reactive. Derivatives with etc. are also much less reactive. Aluminum organyls react with most solvents (exception: saturated and aromatic hydrocarbons), contact with chloroform ( ) can even lead to explosions. Thermal cleavage by dehydroaluminization begins for organyls of aluminum with β-branched alkyl radicals at 80 ° C, for organyls of aluminum with n -alkyl radicals at 120 ° C.
Individual evidence
- ↑ DF Shriver, PW Atkins: Inorganic Chemistry . Oxford University Press, 2006, ISBN 978-0-19-926463-6 .
- ^ M. Witt, HW Roesky: Organoaluminum chemistry at the forefront of research and development. ( Memento of the original from October 6, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: Curr. Sci. 78, 2000, p. 410.
- ^ W. Hallwax, A. Schafarik: About the compounds of earth metals with organic radicals In: Liebigs Ann. Chem. 1859, 109, pp. 206-209, doi : 10.1002 / jlac.18591090214 .
- ^ Christoph Elschenbroich : Organometallics . VCH, Weinheim 2006, ISBN 978-3-527-29390-2 .
- ^ W. Uhl: Organoelement Compounds Possessing Al – Al, Ga – Ga, In – In, and Tl – Tl Single Bonds . In: Adv. Organomet. Chem. . 51, 2004, pp. 53-108. doi : 10.1016 / S0065-3055 (03) 51002-4 .
- ↑ Michael J. Krause, Frank Orlandi, Alfred T. Saurage, Joseph R. Zietz Aluminum Compounds, Organic. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Weinheim 2005, doi : 10.1002 / 14356007.a01_543
- ^ W. Uhl, B. Jana: A persistent alkylaluminum peroxide: surprising stability of a molecule with strong reducing and oxidizing functions in close proximity. In: Chemistry. Volume 14, number 10, 2008, pp. 3067-3071, doi : 10.1002 / chem.200701916 , PMID 18283706 .





![{\ displaystyle {\ ce {[HC (C (CH3) NC6H5) 2] Al (CH (Si (CH3) 3) 2) OOC (CH3) 3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4675e3d1fc95501233e51e41044e74258b70159d)




