Moissanite
| Moissanite | |
|---|---|
| General and classification | |
| other names | |
| chemical formula | SiC |
|
Mineral class (and possibly department) |
elements |
|
System no. to Strunz and to Dana |
1.DA.05 ( 8th edition : I / B.02) 03/01/08/01 |
| Crystallographic Data | |
| Crystal system | hexagonal |
| Crystal class ; symbol | 6 / mm |
| Space group | P 6 3 mc (No. 186) |
| Lattice parameters | a = 3.0810 (2) Å ; c = 15.1248 (10) Å |
| Formula units | Z = 6 |
| Frequent crystal faces | [10 1 0] |
| Physical Properties | |
| Mohs hardness | 9.5 |
| Density (g / cm 3 ) | measured: 3.1 to 3.29; calculated: 3.21 |
| Cleavage | indistinct after {0001} |
| Break ; Tenacity | shell-like |
| colour | colorless, green, emerald green, greenish yellow, bluish, light gray, black |
| Line color | greenish gray, white |
| transparency | transparent |
| shine | Metallic to diamond gloss |
| Crystal optics | |
| Refractive indices |
n ω = 2.616 to 2.757 n ε = 2.654 to 2.812 |
| Birefringence | δ = 0.038 |
| Optical character | uniaxial positive |
| Pleochroism | weak |
Moissanite , chemically also known as carborundum or silicon carbide , is a rarely occurring mineral from the mineral class of the elements. It crystallizes in the hexagonal crystal system with the chemical composition SiC and forms flat, rounded, hexagonal crystals of up to five millimeters in size. The mineral is colorless in its pure state, but due to traces of other elements such as nitrogen , boron or aluminum it shows a wide range of colors from green (nitrogen) through blue to black (aluminum, boron).
Etymology and history
Moissanite was first detected in 1904 by Henri Moissan in a mineral sample from the Canyon Diablo meteorite found near the Barringer Crater . Its composition was first examined in 1892 by François Ernest Mallard and in 1893 by Georges Friedel ; They recognized that it contained a particularly hard material that was inert to hydrochloric acid , and initially believed this to be diamond. In 1904 Moissan was able to examine a large amount of the meteorite and recognized from the typical hexagonal crystals that the meteorite contains silicon carbide. The new mineral was named moissanite after the discoverer.
The artificial production of silicon carbide was first achieved in 1891 by Edward Goodrich Acheson (patented February 1893); moissanite in gemstone quality was first produced in 1997.
classification
In the Strunz system , moissanite is one of the non-metals . After the 8th edition , it forms a group of semimetals and non-metals together with chaoite , diamond, fullerite , graphite and lonsdaleite . In the 9th edition it is the only representative of the non-metal carbides, a subgroup of the non-metallic carbon and nitrogen compounds.
In the Dana system , it forms a separate subgroup of semimetals and non-metals.
Crystal structure
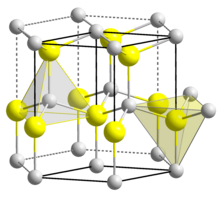
In the most common α-modification, moissanite crystallizes in the hexagonal crystal system in the space group P 6 3 mc (space group no.186 ) with the lattice parameters a = 3.073 Å and c = 15.08 Å as well as six formula units per unit cell . This corresponds to the wurtzite structure.
properties
Moissanite is one of the hardest known naturally occurring substances, only diamond is harder. Like diamond, moissanite is optically transparent, but in contrast to it, it is birefringent .
Moissanite typically crystallizes in hexagonal, tabular crystals. These are flattened along the [10 1 0] plane and rounded at the corners.
The individual crystals in natural occurrences are rarely larger than 1 mm. As of 2014, a 4.1 mm long specimen found in Israel is considered the largest known natural moissanite crystal.
Modifications and varieties
Moissanite occurs in various polymorphic forms. These include various hexagonal, rhombohedral and cubic modifications. The hexagonal moissanite 6H modification is found most frequently, the structure of which corresponds to that of wurtzite . The cubic β-modification (moissanite-3C), which corresponds to the zinc blende structure , also occurs rarely . It was found in the US state of Wyoming . Of the 74 modifications known in man-made silicon carbide, eight are known from nature.
Education and Locations
Silicon carbide forms at the high temperatures that occur in the Earth's mantle or when meteorites hit the earth. The α-modification initially forms at temperatures from 1900 to 2000 ° C. The formation conditions are comparable to those of diamond, so that the two minerals sometimes occur together in kimberlite , such as in Fuxian in the People's Republic of China . If the α-SiC is in contact with elemental silicon at high temperatures and if carbon dioxide is also present, the silicon can react with the carbon dioxide to form β-SiC, which attaches to the α-SiC. Other minerals except diamond, moissanite which is associated, are iron (in meteorites), quartz , garnet , clinopyroxene , Coesite , rutile , graphite, pyrrhotite and cobalt - pyrite (in kimberlite).
Locations include various meteorites, such as the Indarch meteorite in Azerbaijan , the Krymka meteorite in Ukraine and the Canyon Diablo meteorite in the US state of Arizona ; Impact craters like the Nördlinger Ries ; Volcanoes such as the Tolbachik on the Kamchatka Peninsula ( Russia ) and diamond mines, for example in Sakha (Russia) and Kimberley in Western Australia .
use
Due to its rarity, naturally occurring moissanite is not used economically. However, silicon carbide is artificially produced in large quantities from silicon dioxide and carbon . As carborundum, it is an important abrasive , but is also used as a ceramic , insulator and, due to its semiconductor properties, for light-emitting diodes , transistors and varistors .
Highly pure moissanite crystals can be used as a diamond substitute due to their comparable properties. Moissanite has a somewhat lower hardness than diamond, but is thermally more stable in air (up to 1127 ° C, diamond only up to 837 ° C) and is significantly cheaper to manufacture. It is therefore used in experiments under high pressure and high temperature.
See also
literature
- Moissanite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( PDF 61 kB )
- Entry on moissanite. In: Römpp Online . Georg Thieme Verlag, accessed on March 7, 2014.
- Simonpietro Di Pierro, Edwin Gnos, Bernard H. Grobety, Thomas Armbruster, Stefano M. Bernasconi, Peter Ulmer: Rock-forming moissanite (natural α-silicon carbide). In: American Mineralogist . 2003, 88, pp. 1817-1821 ( abstract, pdf ).
- Gian Carlo Capitani, Simonpietro Di Pierro, and Gioacchino Tempesta: The 6H-SiC structure model: Further refinement from SCXRD data from a terrestrial moissanite. In: American Mineralogist . 2007, 92, 403-407 ( abstract, pdf ).
Web links
- Mineral Atlas: Moissanite (Wiki)
- Mindat - Moissanite (English)
- RRUFF Database-of-Raman-spectroscopy - Moissanite (English)
Individual evidence
- ↑ Gian Carlo Capitani, Simonpietro Di Pierro, Gioacchino Tempesta: The 6H-SiC structure model: further refinement from SCXRD data from a terrestrial moissanite . In: American Mineralogist . tape 92 , 2007, p. 403-407 ( rruff.info [PDF; 244 kB ; accessed on July 8, 2017]).
- ↑ a b c d Moissanite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( PDF 61 kB )
- ↑ a b c Moissanite at mindat.org (English)
- ^ Henri Moissan : Nouvelles recherches sur la météorite de Canon Diabolo . In: Comptes rendus . 1904, 139, pp. 773–786 ( text in Gallica , French)
- ↑ Entry on silicon carbide. In: Römpp Online . Georg Thieme Verlag, accessed on March 7, 2014.
- ↑ Patent US492767: A method for making carborundum, an industrial abrasive called also silicon carbide. (PDF 179.4 kB)
- ↑ a b c d Entry on moissanite. In: Römpp Online . Georg Thieme Verlag, accessed on March 7, 2014.
- ^ Emmanuel Fritsch, Vered Toledo, Antoinette Matlins: Record-Size Natural Moissanite Crystals Discovered in Israel. Gemological Institute of America , 2014, accessed November 30, 2017 .
- ↑ J. Bauer, J. Fiala, R. Hrichova: Natural α-silicon carbide . In: American Mineralogist . tape 48 , 1963, pp. 620–635 ( rruff.info [PDF; 795 kB ; accessed on July 8, 2017]).
- ↑ Irene Leung, Wenxiang Guo, Irving Friedman, Jim Gleason: Natural occurrence of silicon carbide in a diamondiferous kimberlite from Fuxian . In: Nature . 1990, 346, 352-354, doi : 10.1038 / 346352a0 .
- ↑ RM Hough, I. Gilmour, CT Pillinger, JW Arden, KWR Gilkess, J. Yuan, HJ Milledge: Diamond and silicon carbide in impact melt rock from the Ries impact crater . In: Nature . 1995, 378, pp. 41-44, doi : 10.1038 / 378041a0
- ↑ Ji-an Xu, Ho-kwang Mao: Moissanite: A Window for High-Pressure Experiments. In: Science . 2000, 290, pp. 783-785, doi : 10.1126 / science.290.5492.783 .