Benzothiazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
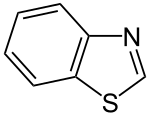
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzothiazole | |||||||||||||||
| other names |
1,3-benzothiazole ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 7 H 5 NS | |||||||||||||||
| Brief description |
colorless to light yellow liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 135.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.246 g cm −3 |
|||||||||||||||
| Melting point |
2 ° C |
|||||||||||||||
| boiling point |
230 ° C |
|||||||||||||||
| Vapor pressure |
0.13 mbar (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.642 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzothiazole is a chemical compound from the group of sulfur - nitrogen - heterocycles .
Extraction and presentation
Benzothiazoles can be obtained by reacting 2-aminothiophenol with acyl chlorides .
properties
Benzothiazole is a colorless to brown, very difficult to ignite liquid with an aromatic odor, which is sparingly soluble in water. It decomposes when heated, producing hydrogen cyanide , sulfur oxides , nitrogen oxides , carbon monoxide and carbon dioxide .

use
Benzothiazole is an interesting carbonyl equivalent. It reacts with aldehydes or ketones to form α-hydroxycarbonyl compounds. The derived compound benzothiazole-2-thiol is used in the rubber industry as a vulcanization accelerator.
Individual evidence
- ↑ a b c d e f g h i j Entry on benzothiazole in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Benzothiazoles at TCI Europe, accessed on June 27, 2011.
- ↑ a b Benzothiazole data sheet from Sigma-Aldrich , accessed on December 30, 2010 ( PDF ).
- ^ TE Gilchrist "Heterocyclic Chemistry" 3rd Edition, Longman, 1992.


