Aminothiophenols
| Aminothiophenols | |||||||||
| Surname | 2-aminothiophenol | 3-aminothiophenol | 4-aminothiophenol | ||||||
| other names |
|
|
|
||||||
| Structural formula |
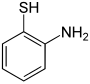
|

|

|
||||||
| CAS number | 137-07-5 | 22948-02-3 | 1193-02-8 | ||||||
| ECHA InfoCard | 100,004,798 | 100.041.207 | 100.013.422 | ||||||
| PubChem | 8713 | 31577 | 14510 | ||||||
| Molecular formula | C 6 H 7 NS | ||||||||
| Molar mass | 125.19 g mol −1 | ||||||||
| Physical state | firmly | liquid | firmly | ||||||
| Brief description | yellow solid or light yellow liquid with an unpleasant odor | ||||||||
| Melting point | 26 ° C | ? ° C | 37-42 ° C | ||||||
| boiling point | 234 ° C | 190 ° C (21 hPa) | 140–145 ° C (21 hPa) | ||||||
| density | 1.17 g cm −3 (20 ° C) | 1.18 g cm −3 | ? g cm −3 | ||||||
| solubility | practically insoluble in water (0.027 g l −1 at 20 ° C) |
? | ? | ||||||
| Refractive index | 1.642 (20 ° C) | 1.656 (20 ° C) | ? | ||||||
|
GHS hazard labeling |
|
|
|
||||||
| H-phrases | 302-314-317-410 | 302-312-314-332 | 314 | ||||||
| P-phrases | 273-280-305 + 351 + 338-310-501 | 280-305 + 351 + 338-310 | 280-305 + 351 + 338-310 | ||||||
| Toxicological data | 25 mg kg −1 ( LD 50 , mouse , ip ) | ? | 320 mg kg −1 ( LD 50 , mouse , oral ) | ||||||
Aminothiophenols (also called aminobenzenethiols or mercaptoanilines ) are aromatic compounds from the group of thiols that are derived from both thiophenol and aniline . The structure consists of a benzene ring with an attached thiol group (-SH) and an amino group (-NH 2 ) as substituents . Their different arrangement results in three constitutional isomers .
Extraction and presentation
2- or 4-aminothiophenol can be produced by reacting 2- or 4-chloronitrobenzene with sulfidic reducing agents. 2-aminothiophenol was first described by AW Hofmann illustrated, it first in the cleavage of a compound with potassium hydroxide discovered that arises when one of benzoyl on Phenyl act or can phenylbenzamide with sulfur heated.
use
2-aminothiophenol can be used to produce 2-substituted benzothiazole derivatives. 4-aminothiophenol can be used to manufacture polymers on nanotubes and drugs such as azalanstate .
Individual evidence
- ↑ a b c d e f Entry on 2-aminothiophenol in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d data sheet 3-aminothiophenol from Sigma-Aldrich , accessed on December 30, 2010 ( PDF ).
- ↑ a b c d data sheet 4-aminothiophenol from Sigma-Aldrich , accessed on December 30, 2010 ( PDF ).
- ↑ Entry on 3-aminobenzenethiol at TCI Europe, accessed on June 27, 2011.
- ↑ a b c data sheet 2-aminothiophenol from Sigma-Aldrich , accessed on December 30, 2010 ( PDF ).
- ↑ Entry on 4-aminobenzenethiol at TCI Europe, accessed on June 27, 2011.
- ^ Process for preparing 2- and 4-aminothiophenols
- ↑ ETH Zurich: Hermann Hauser: On the knowledge of 2-aminophenyl-benzothiazoles
- ↑ Data sheet 2-aminothiophenol from AlfaAesar, accessed on December 30, 2010 ( PDF )(JavaScript required) .
- ↑ Shirō Kobayashi, Helmut Ritter, David Kaplan: Enzyme-catalyzed synthesis of polymers ; ISBN 978-3-540-29212-8 .
- ↑ Daniel Lednicer: Strategies for Organic Drug Synthesis and Design ; ISBN 978-0-47019039-5 .


