Borazine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Borazine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | B 3 H 6 N 3 | |||||||||||||||
| Brief description |
clear, colorless liquid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 80.53 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.83 g cm −3 |
|||||||||||||||
| Melting point |
−58 ° C |
|||||||||||||||
| boiling point |
55.0 ° C |
|||||||||||||||
| solubility |
Decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−541.0 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Borazine ( cyclotriborazane ) is a cyclic compound of boron , nitrogen and hydrogen with the empirical formula B 3 H 6 N 3 . Borazine is isoelectronic to benzene , but the question of the aromaticity of the compound must be discussed critically. According to a suggestion by Nils Wiberg , the basic structure of these compounds is also called “inorganic benzene”. The name borazole , which is not systematically permitted, is derived from this.
The name borazine / borazine was also given to the iminoborane group of substances based on the structurally related alkynes .
history
Borazin was first made by Alfred Stock in the early 20th century. He obtained it by heating diborane and ammonia .
Extraction and presentation
Borazine can be produced by heating a mixture of diborane and ammonia in a molar ratio of 1: 2 to 250-300 ° C. The yield of this chemical reaction is 50%:
Alternatively, as starting materials and lithium borohydride and ammonium chloride can be used, which leads to a higher yield:
Instead of lithium borohydride , you can also use sodium borohydride :
Another borazine synthesis is the following two-step:
The synthesized borazine is then concentrated by distillation.
properties
Borazine decomposes in water to form boric acid , ammonia and hydrogen . Borazine ( enthalpy of formation ΔH f = −531 k J / mol) is thermally very stable.
structure
Borazine is isostatic to benzene. This means that the bonds and bond conditions are the same or very similar to those in benzene. The C -C distance in benzene is 139.7 pm . The bond length between boron and nitrogen in borazine is 143.6 pm. As expected, it lies between the value for a BN single bond (151 pm), as found in boron nitride , and that for a B = N double bond (131 pm).
Mesomerism
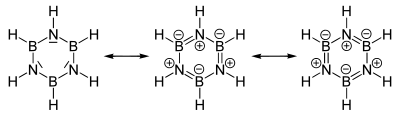
The electronegativity difference between boron (2.04 on the Pauling scale ) and nitrogen (3.04) as well as the lack of electrons on the boron atom and the free electron pair in nitrogen favor the formation of mesomers of the borazine structure. Boron plays the role of a Lewis acid , nitrogen that of a Lewis base .
Reactions
Because of the difference in atoms and thus the polarity of the B – N bonds, borazine is chemically much more reactive than benzene. Borazine reacts easily with polar compounds such as hydrogen chloride , water and methanol . Hydrogen chloride reacts with borazine in an addition reaction . With benzene this reaction would not take place. The reaction with bromine does not require a catalyst .
Aromaticity
As a rule, geometrical (bond length compensation), magnetic (magnetic susceptibility and its anisotropy, ring current effects, NMR shielding) and, above all, energetic properties (aromatic stabilization energy and the associated, unusual chemical behavior) are used as criteria for aromaticity. Borderline cases are difficult to assess because the individual criteria do not always correlate positively with one another and no fixed limits are defined for the assessment of aromatic / non-aromatic. The question of aromaticity is therefore usually discussed on the basis of comparative examples, with benzene serving as the undisputed reference in many cases.
Compared to benzene, borazine is characterized by an uneven π-electron distribution (electronegativity difference boron / nitrogen, accumulation of electron density on nitrogen). While the free borazine is still characterized by a planar structure with balanced bond lengths, tricarbonyl-chromium complexes, in contrast to the benzene analogues, are no longer planar (preferred coordination via the nitrogen in the borazine). Aromatic stabilization energies (ASE), magnetic susceptibility exaltation (Λ) and NICS values ( nucleus independent chemical shift ) of borazine calculated on B3LYP / 6-31G * result in values that are significantly lower than that of benzene, but the ASE in particular is still roughly half as large as in the case of the clearly aromatic reference.
| ASE kJ / mol | Λ | NICS | |
|---|---|---|---|
| benzene | 175.4 | −16.7 | −11.5 |
| Borazine | 40.2 | −5.9 | −2.1 |
More detailed investigations of the magnetic properties of borazine confirm its complex topology, which makes the simplified discussion based on Λ and NICS values less meaningful, and ascribing borazine based on the magnetic properties π-aromaticity, but no "global" aromatic character. The cyclic electron delocalization is described by the authors as "ineffective". Reactions in the gas phase are similar to the electrophilic substitution reaction pattern typical of benzene.
The discussion about the aromatic character of borazine does not seem to have been concluded among experts at the moment, but the basic trend is that borazine has a certain aromatic character, even if it is significantly less aromatic than that of benzene.
use
Borazine and its derivatives are of interest as potential precursor products on the way to boron nitride - ceramics . According to recent calculations, mixed amino-nitro-substituted borazines promise significantly higher detonation speed , detonation pressure and Gurney energy than conventional explosives such as B. CL-20 .
swell
- ↑ a b c Entry on Borazine. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1116.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 1,3,5,2,4,6-triazatriborinane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 19, 2019, is reproduced from a self-classification by the distributor .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-6.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, pp. 548-549, ISBN 3-342-00280-8 .
- ^ Paul von Ragué Schleyer , Haijun Jiao: What is Aromaticity? In: Pure and Applied Chemistry . Vol. 68, No. 2, 1996, pp. 209-218, doi : 10.1351 / pac199668020209 .
- ↑ Gottfried Huttner , Bernhard Krieg: Crystal and molecular structure of tricarbonyl (hexaäthylborazin) chrom (0). In: Chemical Reports . Vol. 105, No. 10, 1972, pp. 3437-3444, doi : 10.1002 / cber.19721051031 .
- ^ Hyp J. Dauben Jr., James Dennis Wilson, John L. Laity: Diamagnetic Susceptibility Exaltation as a Criterion of Aromaticity. In: Journal of the American Chemical Society . Vol. 90, No. 3, 1968, pp. 811-813, doi : 10.1021 / ja01005a059 .
- ^ Paul von Ragué Schleyer, Christoph Maerker, Alk Dransfeld, Haijun Jiao, Nicolaas JR van Eikema Hommes: Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. In: Journal of the American Chemical Society. Vol. 118, No. 26, 1996, pp. 6317-6318, doi : 10.1021 / ja960582d .
- ↑ Eluvathingal D. Jemmis, Boggavarapu Kiran: Aromaticity in X 3 Y 3 H 6 (X = B, Al, Ga; Y = N, P, As), X 3 Z 3 H 3 (Z = O, S, Se) , and Phosphazenes. Theoretical Study of the Structures, Energetics, and Magnetic Properties. In: Inorganic Chemistry . Vol. 37, No. 9, 1998, pp. 2110-2116, doi : 10.1021 / ic970737y .
- ↑ Rafael Islas, Eduardo Chamorro, Juvencio Robles, Thomas Heine, Juan C. Santos, Gabriel Merino: Borazine: to be or not to be aromatic. In: Structural Chemistry . Vol. 18, No. 6, 2007, pp. 833-839, doi : 10.1007 / s11224-007-9229-z .
- ↑ Barbara Chiavarino, Maria Elisa Crestoni, Annito Di Marzio, Simonetta Fornarini, Marzio Rosi: Gas-Phase Ion Chemistry of Borazne, an Inorganic Analogue of Benzene. In: Journal of the American Chemical Society. Vol. 121, No. 48, 1999, 11204-11210, doi : 10.1021 / ja992220m .
- ^ Ernst-Christian Koch, Thomas M. Klapötke : Boron-Based High Explosives. In: Propellants, Explosives, Pyrotechnics. Vol. 37, No. 3, 2012, pp. 335-344, doi : 10.1002 / prep.201100157 .







